Aude Clapies and Bruno Brisson (Poietis, France) and Markus Nuopponen (UPM, Finland)
INTRODUCTION
GrowInk™ bioinks are animal free, ready to use hydrogels that can be mixed directly with cells for 3D bioprinting applications. The two main components in GrowInks are nanofibrillar cellulose (NFC) and water, providing a fully defined and customizable matrix. GrowInks mimic the in vivo environment supporting cell growth and differentiation. The stiffness of GrowInks can be tuned to match to the requirements of specific cells. GrowInks are room temperature stable and can be used with or without the cross-linking agent depending on the application, but all enable cell printing layer-by-layer with high-precision cell positioning.
In this study, the printability and cell compatibility of GrowInk-N and GrowInk-T with the bioprinter NGB-R™ from Poietis (Fig 1.) were evaluated. Based on 10+ years’ experience of innovative research, NGB-R is a 4th generation multi-modal biofabrication instrument that uses unique laser-assisted bioprinting technology. Both extrusion printing and laser printing of GrowInk-T were studied for fabrication of layered fibroblast/keratinocyte cell models to investigate the suitability of the bioinks for printing and thus tissue engineering applications.

Figure 1. NGB-R bioprinter from Poietis.
MATERIALS
- GrowInk-N (UPM, Finland)
- GrowInk-T (UPM, Finland)
- Cell culture media
- Fibroblast medium
- Keratinocyte medium
- Cells
- Fibroblasts (from primary culture)
- Keratinocyts (from primary culture)
- H&E stain (Hematoxylin and eosin stain)
- Goldner stain
METHODS
1.Printability by laser-assisted bioprinting
- GrowInk-N was diluted 3:2 with ultra pure water, and further 2:13 with fibroblast culture medium.
- GrowInk-T was diluted 1:1 with ultra pure water and further 1:4 with fibroblast culture medium.
- Laser printing allows cell distribution with micro-scale resolution (single-cell) and precision of 10µm to be achieved to better replicate biological tissues with higher levels of detail and cell viability (>95%).
2.Printability by extrusion
- GrowInk-N was diluted 3:2 with ultra pure water.
- GrowInk-T was diluted 1:1 with ultra pure water.
- Printed by pneumatic extrusion layer-by-layer.
- The printed object was square with side length of 12 mm.
- Each layer was 500 µm in thickness, resulting in a total thickness of approximately 2 mm.
- Cell compatibility assay
- For the cell compatibility assay, GrowInk-T hydrogel, diluted 1:1 with ultra pure water, was used with layer-by-layer extrusion printing as described above.
- Cells were printed by laser (Fig. 2).
- Fibroblasts : 10,000 cells per layer between the GrowInk-T layers.
- Keratinocytes : 400,00 cells per layer on top of the printed structure.
- Gel layers and fibroblasts were printed on day 0 and incubated in fibroblast media.
- Keratinocytes were printed on day 3 and incubated in keratinocyte media.
- On day 5, the gel was analyzed by histology process with H&E and Goldner staining.
- Tissues were fixed, dehydrated and embedded in paraffin wax, the resulting block was mounted on a microtome and cut into thin slices. The slices were fixed to microscope slides and rehydrated.
- H&E stain: Hematoxylin colors the nuclei of cells blue, eosine stains the cytoplasm and extracellular matrix in shades of pink.
- Goldner staining: Cell cytoplasm were stained in red, collagen in green and nuclei in black.
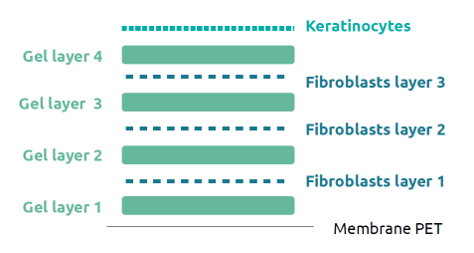
Figure 2. Schematic representation of the 3D model printed with GrowInks. Gel layers were printed by extrusion and the cells were printed by laser.
RESULTS
Both GrowInk-N and GrowInk-T hydrogels were easy to print by laser. Small droplets (< 200 µm in diameter) were printed reproducibly with high precision (Fig. 3). Likewise, GrowInk-N and GrowInk-T were also succesfully printed by pneumatic extrusion. GrowInk-N was slightly more viscous than GrowInk-T, which resulted in slightly better structural stability of the objects printed. Both bioinks remained stable over time (Fig. 4).
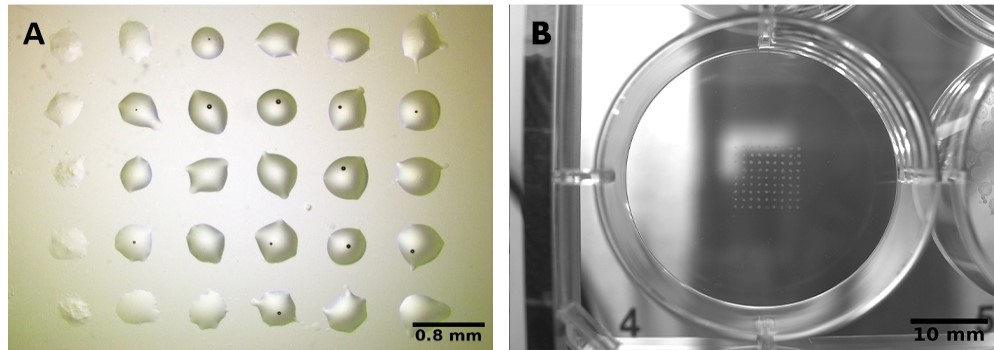
Figure 3. GrowInks printed by laser, A) GrowInk-N and B) GrowInk-T. 200 µm droplets were printed reproducibly with high precision.
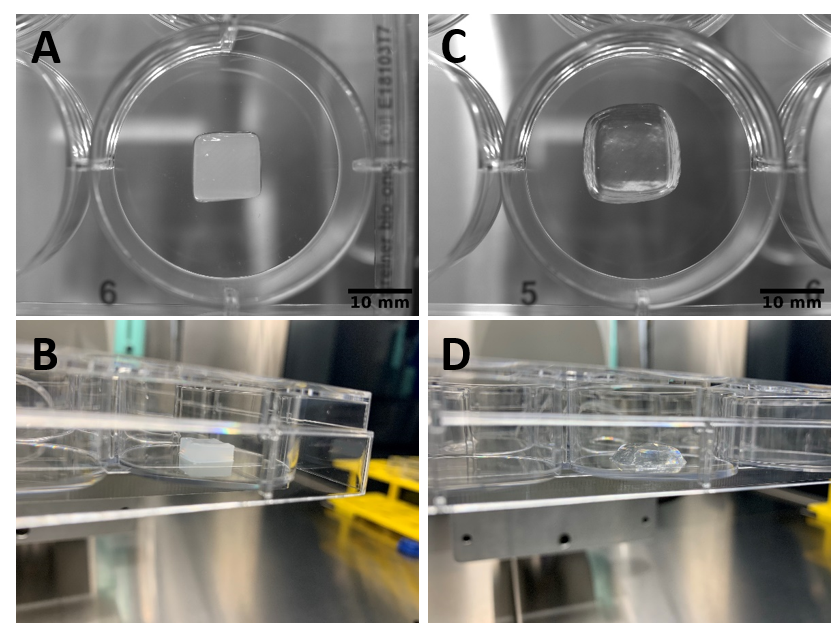
Figure 4. GrowInks printed by extrusion. GrowInk-N (A and B) and GrowInk-T (C and D).
Cell compatibility assay with GrowInk-T showed that the bioink was not stained by Goldner, however it could be visualized with polarizing microscope filter. GrowInk-T was stained by Saffran from the HE stain, suggesting that GrowInk-T is similar to the connective tissue. Fibroblasts were not observed in the 3D printed dermis, which was thought to be as a consequence of the small number of cells compared to the quantity of gel. Laser-printed keratinocytes were found to appreciate the environment on top of 3D printed GrowInk-T. The cells were viable and well structured (Fig. 5). With more maturation of the model it is highly likely that an epidermis would have emerged.
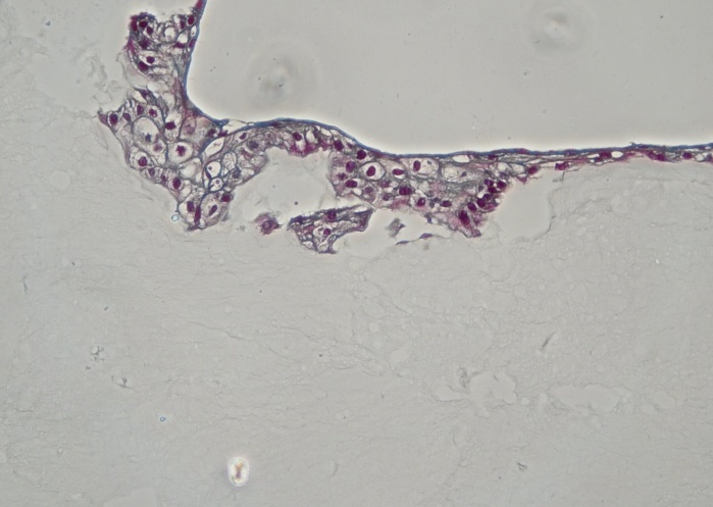
Figure 5. Histological characterization of the 3D printed model construct. Stained by Goldner, showing the structured morphology of keratinocytes on top of GrowInk-T.
CONCLUSIONS
GrowInk-N and GrowInk-T are both easy to use in laser- and extrusion 3D printing with the Poietis bioprinter NGB-R. GrowInk properties make them ideal 3D printing matrices; the shear-thinning property enables easy, clog-free printing of stable structures at room temperature, and the stiffness can be tuned for specific cell types. Fibroblasts were successfully laser-printed between the extrusion-printed GrowInk-T layers, and the keratinocytes printed on top of GrowInk-T showed correct morphology and good viability. GrowInks have high potential for various applications in bioprinting and tissue models in comparison with conventional cross-linked 3D hydrogels and scaffolds, as GrowInks are ready-to-use and stable, and the absence of animal-derived components opens up new possibilities for the bioinks to better recapitulate the ECM environment in tissue engineering.