Jonathan Sheard, Ph.D., Sheard BioTech Ltd & University of Reading, United Kingdom
Introduction
Culture expansion of cells in 3D versus the classical 2D approach is proving to be an invaluable tool for use in assay development, for use in drug screening, as well as providing a clearer understanding of cell behaviour in cell-based regenerative therapies [1, 2]. Notably, mesenchymal stem cells (MSCs) are the most researched cells for their potential use in cell-based therapies. As such, it is crucial to fully characterise these cells within novel 3D environments prior to their use and application for such therapies. It has been previously shown that cell seeding density and the 3D cell carrier affect the behaviour and differentiation capacity of bone marrow derived and umbilical cord/Wharton's jelly derived MSCs towards bone and cartilage regeneration respectively [3, 4, 5].
Here, adipose derived MSCs (ADMSCs) were cultured in nanofibrillar cellulose, GrowDex®, under various conditions for 1 week to characterise their behaviour ahead of use in the development of further cell based assays.
Materials
- Adipose Derived Mesenchymal Stem Cells (ADMSCs, Cat# PT-5006, Lonza)
- Complete Media: DMEM (High Glucose) supplemented with 10% FBS, 1% Pen/Strep and 1% L-Glutamine (Sigma Aldrich)
- 96-well non-treated tissue culture plates (Cat# 83.3924.500, Sarstedt)
- GrowDex, 1.5% (Cat# 100 103 005, UPM)
- EVOS Live imaging system (ThermoFisher)
- Image-J image analysis software (NIH)
Method
- At all times, ADMSCs were cultured in complete media and incubated at 37°C with 10% CO2.
- Following trypsinization, cells were resuspended in media at a concentration of 1 x 106 cells/ml.
- Cells were then mixed with GrowDex and the appropriate volume of media added to provide final concerntrations of 0.1%, 0.2% or 0.4% GrowDex seeded with cell densities of 250, 500 or 1000 cells/µl.
- 100 µl of each of sample were pipetted in triplicate into wells of a 96-well non-tissue culture plate.
- 50 µl of media was pipetted carefully on top of the cell/GrowDex mix.
- The plate was incubated at 37°C with 10% CO2 with culture media exchange being performed every 2 to 3 days.
- Images of the samples were taken using the EVOS imaging system using 4x and 10x objectives. Images captured using the 4x objective were loaded into image J where the number and area of the cell spheres was measured and quantified.
Results
Following 7 days culture expansion of the ADMSCs at 37°C, cell spheres were visible in all sample wells. The spheres varied in both size and number depending on the initial cell seeding density and the concentration of GrowDex used for the culture.
Small spheres were observed in all wells irrespective of the GrowDex concentration or the initial cell seeding density used (Fig.1-2). The number and size of spheres was quantified for each sample and it was found that the majority of spheres had an average size less than 500 µm2(Fig.3). However, in 0.1% GrowDex larger spheres ranging from 500 – 200,000 µm2 were also observed at cell seeding densities of 250 – 500 cells/µl. At the highest cell concentration (1,000 cells/µl) and 0.1% GrowDex a very large sphere, size >200,000 µm2, was present in all three wells (Fig.1 C & F)
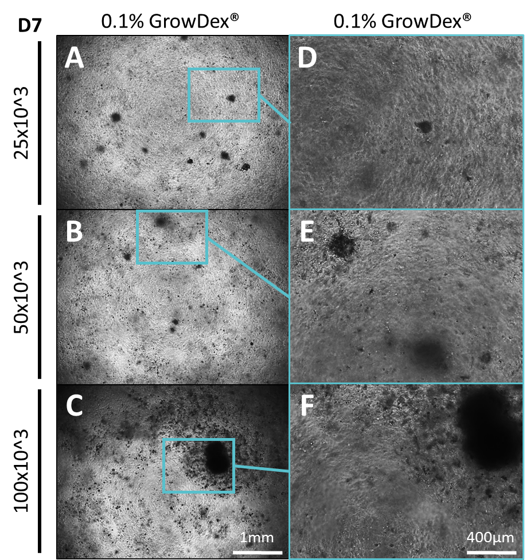
Figure 1. Primary ADMSCs were seeded at different cell densities within different concentrations of GrowDex and cultured for 7 days. Cells were seeded at densities of 250, 500 or 1000 cells/µl within GrowDex to a final concentration of 0.1%. Images were captured using 4x (A-C) and 10x (D-F) magnification.
Sphere formation was observed in the 0.2% GrowDex samples at all cell seeding densities (Fig.2 G-I). However, it was noted that fewer spheres were present in the 0.2% samples and their area was smaller when compared to the 0.1% GrowDex samples (Fig.3 A-C).
Within 0.4% GrowDex, the number of spheres were seen to increase relative to the seeding density, from 250 to 1000 cells/µl. However, these spheres showed little variation in sphere area (Fig.2 J-L, Fig.3 A & D).
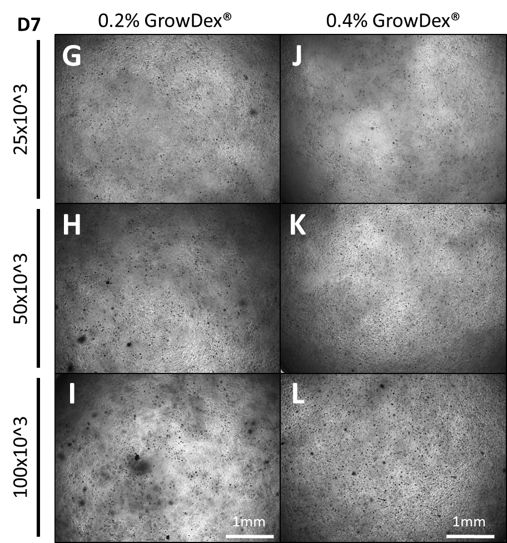
Figure 2. Primary ADMSCs cultured for 7 days in GrowDex at concentrations of 0.2% (G-I) and 0.4% (J-L) with differing initial cell seeding densities.
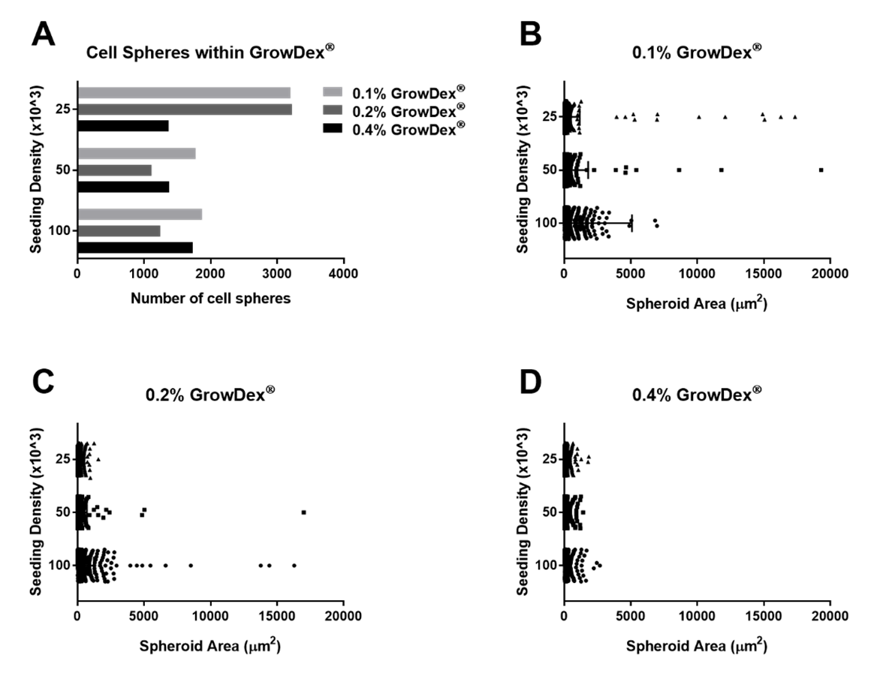
Figure 3. Quantification of total cell spheres from images collected in 100µl of 0.1% GrowDex, 0.2% GrowDex and 0.4% GrowDex (A). Quantification of the area of the spheres (µm2) observed in 0.1% GrowDex (B), 0.2% GrowDex (C) and 0.4% GrowDex (D). Cells were seeded at 250 (top row data), 500 (middle row data) and 1000cells/µl (bottom row data*). *The area of the largest spheres (>200,000µm2) were excluded from the graphs.
Conclusions
Differences in seeding density is known to impact cell bahaviour. Notably, MSCs isolated from Wharton's jellys were shown to have higher glycosaminoglycan and collagen contents when seeded at densities of 25 to 50 thousand cells/µl on a non-woven polyglycolic acid scaffold [4]. Additionally, bone marrow MSCs seeded onto a copolymer scaffold at higher cell seeding densities formed more bone than those seeded at lower densities following osteogenic induction [3].
Aggregated MSC spheres have also been shown to provide enhanced osteogenic and adipogenic differentiation potential [6], as well as produce higher amounts of cartilage matrix deposition [7]. Additionally, conditioned media harvested from MSCs cultured as spheres showed stong anti-inflammatory effects [8, 9, 10]. Therefore, it is important to characterise the behaviour and potential application of cells embeded within this novel 3D nanofibrillar cellulose, GrowDex.
From the results shown here, it can be easily seen that different cell seeding densities along with variable concentrations of GrowDex can affect cell behaviour after 7 days of cell culture; notably the formation cell spheres. However, other affects are yet to be determined.
References
- Malinen, M. M., et al. (2014). "Differentiation of liver progenitor cell line to functional organotypic cultures in 3D nanofibrillar cellulose and hyaluronan-gelatin hydrogels." Biomaterials 35(19): 5110-5121.
- Lou, Y.-R., et al. (2014). "The Use of Nanofibrillar Cellulose Hydrogel As a Flexible Three-Dimensional Model to Culture Human Pluripotent Stem Cells." Stem Cells and Development 23(4): 380-392.
- Yassin, M. A., et al. (2015). "Cell seeding density is a critical determinant for copolymer scaffolds‐induced bone regeneration." Journal of Biomedical Materials Research. Part a 103(11): 3649-3658.
- Wang, L., et al. (2009). "Effect of Initial Seeding Density on Human Umbilical Cord Mesenchymal Stromal Cells for Fibrocartilage Tissue Engineering." Tissue Engineering. Part A 15(5): 1009-1017.
- Vo, T. N., et al. (2016). "Effects of Cellular Parameters on the In Vitro Osteogenic Potential of Dual-Gelling Mesenchymal Stem Cell-Laden Hydrogels." Journal of biomaterials science. Polymer edition 27(12): 1277-1290.
- Frith, J. E., et al. (2010). "Dynamic three-dimensional culture methods enhance mesenchymal stem cell properties and increase therapeutic potential." Tissue Eng Part C Methods 16(4): 735-749.
- Suzuki, S., et al. (2012). "Properties and usefulness of aggregates of synovial mesenchymal stem cells as a source for cartilage regeneration." Arthritis Research & Therapy 14(3): R136-R136.
- Bartosh, T. J., et al. (2010). "Aggregation of human mesenchymal stromal cells (MSCs) into 3D spheroids enhances their anti-inflammatory properties." Proceedings of the National Academy of Sciences of the United States of America 107(31): 13724-13729.
- Bartosh, T. J. and J. H. Ylostalo (2014). "Preparation of anti-inflammatory mesenchymal stem/precursor cells (MSCs) through sphere formation using hanging drop culture technique." Current protocols in stem cell biology 28: Unit-2B.6.
- Ylöstalo, J. H., et al. (2012). "Human mesenchymal stem/stromal cells (hMSCs) cultured as spheroids are self-activated to produce prostaglandin E2 (PGE2) that directs stimulated macrophages into an anti-inflammatory phenotype." Stem cells (Dayton, Ohio) 30(10): 2283-2296.