Jenna James, Joseph Lloyd, Matt Dunn, Daniel Merryweather, Rosemary Fricker, Paul Roach Institute for Science and Technology in Medicine, Keele University, United Kingdom
Introduction
Growing cells in 3D has become important in the development of in vitro fabricated tissue. Developing so-called 'organ on a chip' models allows us to mimic how tissues function in a smaller, well defined engineered tissue construct. Various models have been established to replicate many different organ and tissue types, although the central nervous system remains a challenge due to the many different intercommunicating cell types and the dependence of their complex organisation for normal function. The need to develop such complex functional models, particularly using human-relevant tissues (non-animal models) will be highly relevant for establishing a better understanding of neurological dysfunction and an enabling technology for clinical intervention, such as regenerative medicine and cell therapies.
Typical in vitro models of neuronal circuits have so far been achieved by growing cells in a 2D monolayer, being segregated and directed to form a cellular circuit using micro-structures. It is well known that cells grown in 2D do not replicate effectively the activity or function of native cells experiencing a 3D environment, lacking many of the intercellular and environmental cues that guide standard metabolic behaviour in vivo. Here we have established a method of introducing the GrowDex nano-cellulose hydrogel into micro(fluidic) devices fabricated using soft lithography for the trivial conversion of a 2D culture environment to a 3D construct. These devices present micro-channels into which neuronal cell bodies are too large to enter, but neurites are directed along their length to connect to neighbouring cells on the opposite side. We have been able to embed and cast micro-structures down to sub-5 microns in width and height (aspect ratio of 1) with lengths upwards of 800 microns.
Materials & method
- Molds for microfluidic devices (Fig.1) were fabricated using conventional lithographic processing of SU8 to form an inverse master, which was replicated in poly(dimethyl siloxane) PDMS.
- Devices were then fabricated by pouring well-mixed PDMS over the molds in a 10:1 ratio of polymer to cross-linker.
- After two hours at 50 °C the PDMS hardened sufficiently to be peeled from the mold.
- Loading ports were made by coring a small hole above the channels using a 3-gauge corer.
- If casting and lift off of the PDMS was required, a pre-treatment of hydrophobic silane was used to cover the PDMS at this stage (e.g. chemical vapor deposition of hexamethyldisilazane at R.T. for 1 hour). This ensured reduced interaction of the GrowDex® with the PDMS allowing lift-off without damage to the micro-structure. If GrowDex was to left embedded in the structure then this stage was not needed.
- Wettability was confirmed (WCA > 100°) using sessile droplet shape.
- The patterned PDMS was then adhered to a cleaned glass slide, oxygen plasma etched at 30W for 30 seconds in a 300 mtor oxygen atmosphere. This step could be replaced with repeat washes of the glass in tween, water and isopropanol, being dried under clean nitrogen immediately prior to contact with the PDMS.
- A 1 % w/v solution of GrowDex hydrogel was prepared using a DMEM-F12 based culture medium containing 10 % v/v human serum, 1 % L-glutamine, and 1 % pen/strep. A suitable volume of media was first loaded into an Eppendorf tube followed by the required quantity of stock GrowDex solution. It was found that loading in this manner greatly reduced bubble formation and that the hydrogel set significantly faster in the presence of media compared to PBS.
- The hydrogel solution was introduced into the devices using an 18G syringe needle and allowed to fill the microfluidic channels via capillary action at room temperature, Fig 1.
- After one hour each device was filled with culture medium and incubated at 37 °C for ten days, replenishing media lost to evaporation on alternating days.
- If cell culture was required within the device, cells were mixed with GrowDex solution prior to injection.
- If cast features were required, the PDMS was carefully lifted off after gelation to reveal the hydrogel features standing proud of the glass surface, Fig b.
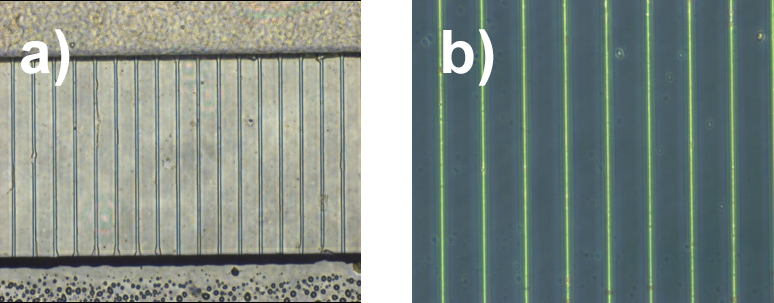
Fig 1. Microscope images of 1 %w/v GrowDex hydrogel a) cast and retained within micro-channels and b) cast and mould removed, after standing at 37 °C for ten days with media replenishment on alternating days. Insert in b) shows schematic representation of cast gel structures.
Results
Features cast in GrowDex were stable for long periods both cast within the PDMS device and presented alone on a glass substrate. Features were well replicated, with porosity of the hydrogel not presenting an issue at this size scale. A neuronal cell line (SH-SY5Y) and primary cortical neurons (E16) were cultured within the structures showing good viability over several days, along with neurite outgrowth where media supplements were used to allow this (retanoic acid for cell lines support this).
Conclusions
We report a protocol for the replication of micro-structures with hydrogel. A 1 % v/w solution of GrowDex was suitable to allow its injection into a microfluidic device, to replicate feature structure and sustain cell culture. Determining the most effective mixing technique (such as the order in which media and gel were mixed) and the equipment that should be used also formed a key part of the resulting protocol.