Tejal V. Pant, Ratnesh Jain, Institute of Chemical Technology, Mumbai, India
Introduction
2D cell culture models are routinely used for various cell culture assays including drug screening and toxicity studies, however they provide limited cell-cell and cell-ECM interactions. Spheroids, grown in 3D environment, show organizational similarities to tumor and can thus be used a relevant model for such studies. Here we have used GrowDex® cell culture matrix for development of spheroids using lung adenocarcinoma cell line A549.
Materials
- A549 cell line (procured from National Center for Cell Sciences cell repository)
- DMEM F12 (HiMedia) supplemented with 10 % FBS (Gibco)
- Non treated tissue culture plates (HiMedia)
- GrowDex 1.5 % (Cat No. 100 100 500, UPM)
- DAPI (Invitrogen), CellVue® Maroon Cell Labeling Kit (eBioscience)
- Live/Dead viability/cytotoxicity kit (Invitrogen)
- GrowDase™ 10 mg/ml (Cat No. 900 102 002, UPM)
Method
- 0.5 % GrowDex was prepared by diluting the stock solution with culture medium. 100 µl of this diluted stock was then transferred to a 96 well plate and incubated for 30 min at 37°C.
- A549 cells were grown in a monolayer in tissue culture treated flask with DMEM F12 supplemented with 10 % FBS.
- At 75-80 % confluency, cells were trypsinized using Trypsin Phosphate Versene Glucose Solution and 100 µl of cell suspension containing 0.5 x 105 cells was added on top of the GrowDex dispensed in the well plate.
- Cells were incubated at 37°C and 5 % CO2 for 15 d. Medium changes were performed every 2-3 days. During medium change, 70 µl of supernatant was removed and 100 µl fresh medium added. Care was taken to avoid disruption of the GrowDex layer.
- Spheroids were recovered from the matrix using GrowDase enzyme subsequent to overnight incubation at 37°C.
- Spheroids were stained for nucleus and membrane using DAPI (Invitrogen) and CellVue® Maroon Cell Labeling Kit (eBioscience) respectively and observed under confocal laser scanning microscope, Leica. Cell viability was determined using Live/Dead viability/cytotoxicity kit. Dilution and staining was performed according to manufacturers instructions.
- Cell viability was also assessed by Alamar Blue assay (Invitrogen). 10 % of dye solution was prepared in complete medium and incubated with cells for 4 h at 37°C. Supernatant was then transferred to 96 well plate and absorbance at 570 nm and 600 nm was recorded to calculate reduction in dye (reduction in dye corresponds to cell viability).
Results
Microscopic observation and simultaneous Live/Dead assay shows that the spheroids start formation by day 3 and continue to grow to a size of approximately 100 µm by day 15. They remain viable till the end of culture and are only slightly less viable than their 2D counterparts with highest viability on day 5 (as seen in graph).
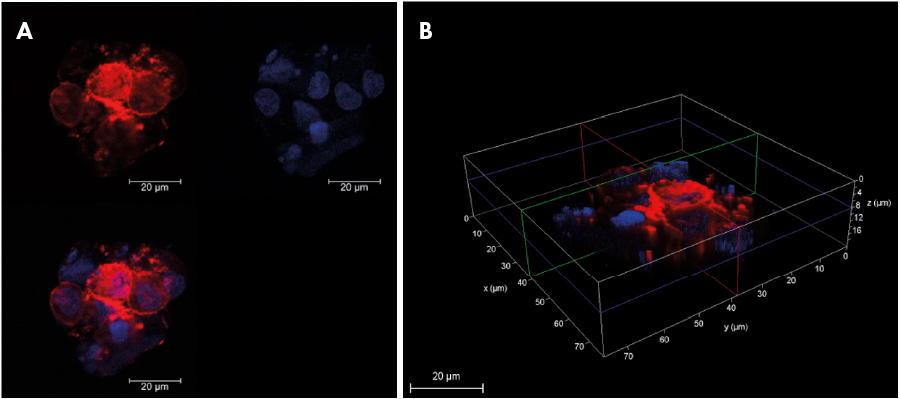
Fig 1. Cell membrane and Nucleus of A549 spheroid stained with Cell Vue Maroon (red) and DAPI (blue) as observed using Confocal microscope (A) Individual channel and merged image in 2D (B) 3D stack showing cross section of spheroid. (Scale bar: 20 µm).
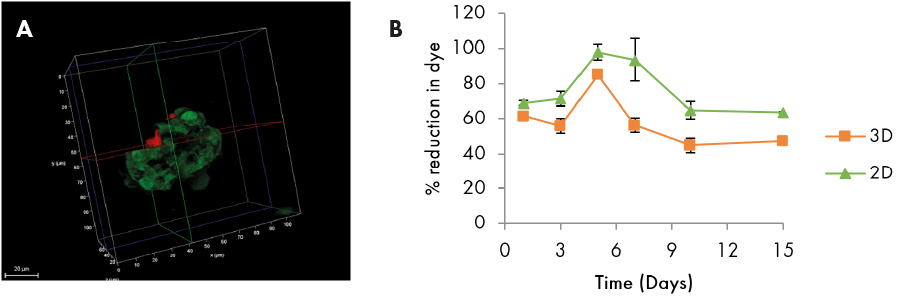
Fig 2. (A) 3D stack of individual spheroid showing live (green) and dead (red) cells. (B) AlamarBlue assay showing percent reduction in dye in monolayer and spheroid culture.
Conclusions
In conclusion, GrowDex provides an easy to use 3D support for development of A549 spheroids. Suitable optical properties of this matrix enable facile visualization of spheroids microscopically. The spheroids can be used for assessing toxicity of different formulations used for drug delivery. These can also be used in combination with other types of lung cells, fibroblasts and/or macrophages, for co-culture studies.
References:
Recent advances in three-dimensional multicellular spheroid culture for biomedical research, Biotechnol. J. 2008, 3, 1172–1184