T. Joki1, L. Ylä-Outinen1, L. Paasonen2, S. Narkilahti1
1 NeuroGroup, BioMediTech, University of Tampere, BioMediTech, Tampere, Finland
2 UPM-Kymmene Oyj, Helsinki, Finland
Introduction
Human stem cell based neuronal cultures are promising tool for studying e.g. disease mechanisms, drug response or developmental biology in vitro. However, traditional flat 2D cultures on top of a rigid surface fail to reproduce in vivo like brain complexity, like high cell density and connectivity with surrounding cell-cell and cell-extra cellular matrix (ECM) contacts. Due to these aspects, the 2D culture can lead to unnatural cell polarization on the flat culture. Different types of 3D cell cultures aim to overcome some of these limitations, by offering cells artificial extracellular matrix (ECM) and more in vivo mimicking environment. The potential of GrowDex for 3D culturing of various cell types has already been demonstrated (Lou et al. 2014; Bhattacharya et al. 2012). It mimics native soft tissue ECM in fiber size and in mechanical properties, thus providing cells a more in vivo like growth environment. In this study pre-differentiated human pluripotent stem cell derived neurons where encapsulated within GrowDex and cultured.
Materials and methods
Human embryonic stem cell (hESC) derived neuronal cells encapsulated in GrowDex (UPM Kymmene Oyj, Finland) were cultured for two weeks. Studied GrowDex concentrations were 1.50 and 1.0 wt%. Two different hydogel volumes, 80 and 60 μl, were studied using a 96-well plate format. Human neurons were derived from hESC line Regea 08/023. Briefly, stem cells were pre-differentiated for eight weeks using previously published method (Lappalainen et al. 2010). The cell concentration used in GrowDex cultures was five million cells per ml of hydrogel. The formation of the neuronal networks inside the hydrogel was evaluated by immunocytochemical staining against neuronal markers Microtubule-Associated Protein 2 (MAP-2) and β-Tubulin III (β-Tub), as previously described (Koivisto et al. 2017). Immunocytochemical samples were imaged with an Olympus IX51 inverted and Zeiss LSM 780-confocal microscope. Image processing was performed using Adobe Photoshop CS4, Huygens Essential and ImageJ –softwares. Confocal data was visualised as shown in Fig. 1.
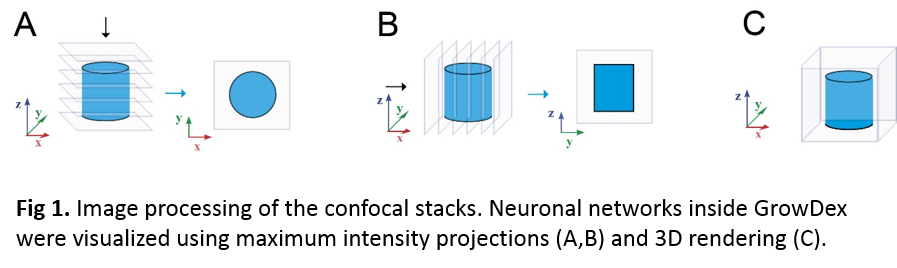
Results
1. GrowDex was stable 3D matrix
Sample preparation, cell plating, cell culture, and analysis were successful with GrowDex. The smaller GrowDex volume caused some loss of the material during washes in the immunocytochemical staining. Overall, the samples were stable during cell culture and analysis (Fig. 2).
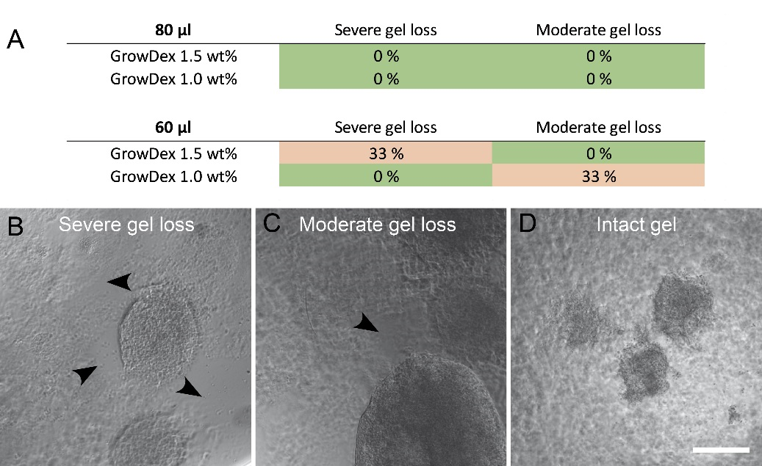
Fig 2. Samples with gel loss as percentage (A) in both studied GrowDex volumes and figures describing the classification (B,C,D). Arrow heads show areas of gel loss. Images were taken after immunocytochemical staining and mounting. Scale bar is 200 μm.
2. GrowDex volume affected cell growth
Neurite outgrowth was analysed from the immunocytochemically stained samples. Cultures were classified into three categories: 1) robust neurite outgrowth, 2) moderate neurite outgrowth, or 3) neural aggregates (no outgrowth), according to the amount of visible neurites (Fig. 3). Smaller hydrogel volume was more supportive of neurite outgrowth whereas the larger volume supported neural aggregates.
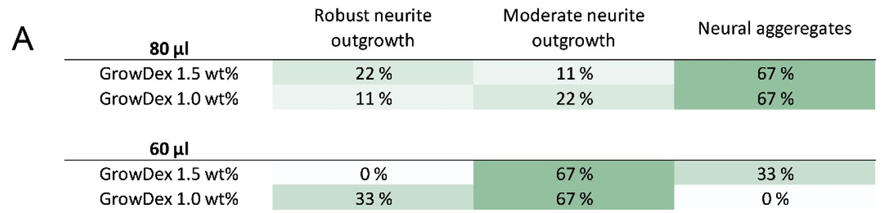
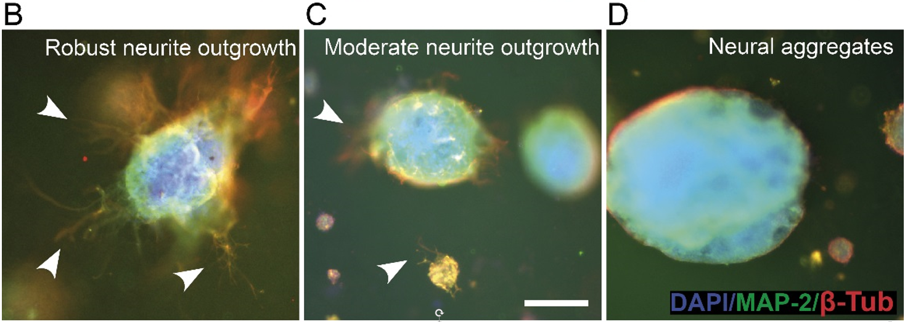
Fig 3. Evaluation of neurite outgrowth in 3D cultures (A) and examples of classification (B,C,D). Arrow heads show areas with neurite outgrowth. Scale bar is 200 μm.
3. Robust neurite outgrowth in 3D
Neurite outgrowth was studied in more detail using confocal imaging. Both GrowDex concentrations supported robust neurite outgrowth in all directions (Fig. 4), in hydrogel volume of 60 μl.
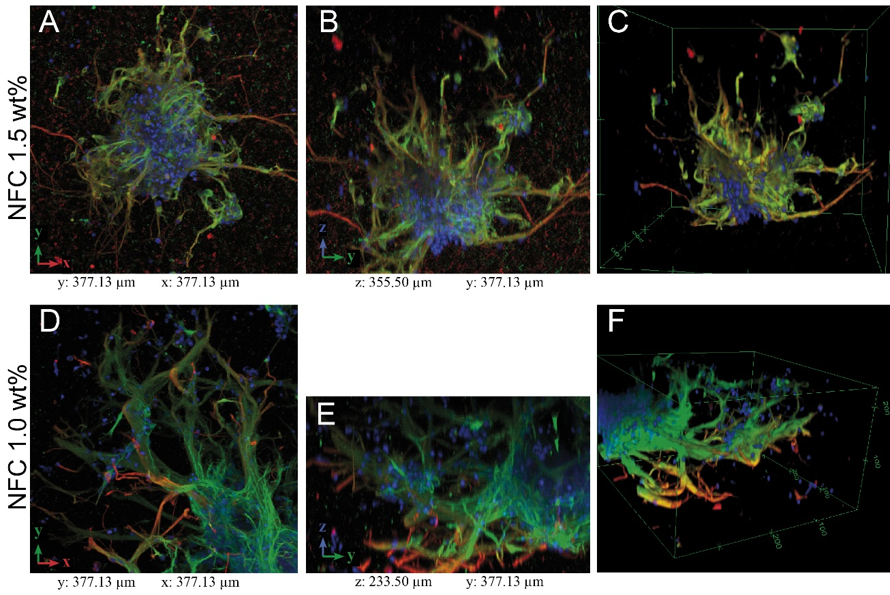
Fig 4. Maximun intensity projections (A,B,D,E) and 3D rendering (C,F) of confocal data (see Fig. 1 for the method). GrowDex concentrations: 1.50 wt% (A,B,C) and 1.0 wt% (D,E,F). Scale bar is 100 μm.
Conclusions
Based on the results it can be concluded that GrowDex offers a very good 3D growth environment for human neural cells. No clear differences were seen between the two studied hydrogel concentrations, whereas the hydrogel volume was found to have an unexpected effect on neurite outgrowth. In larger hydrogel volume (80 μl) the growth as organoid-like aggregates was favored and smaller hydrogel volume (60 μl) supported neurite outgrowth and cell infiltration. These findings suggest that GrowDex is a promising matrix for 3D human neural in vitro models.
References
Bhattacharya, M. et al., 2012. Nanofibrillar cellulose hydrogel promotes three-dimensional liver cell culture. Journal of Controlled Release, 164(3), pp.291–298. Available at: https://doi.org/10.1016/j.jconrel.2012.06.039
Koivisto, J.T. et al., 2017. Bioamine-crosslinked gellan gum hydrogel for neural tissue engineering. Biomedical Materials, pp.1–38. Available at: https://iopscience.iop.org/article/10.1088/1748-605X/aa62b0
Lappalainen, R.S. et al., 2010. Similarly derived and cultured hESC lines show variation in their developmental potential towards neuronal cells in long-term culture. Regenerative medicine, 5(5), pp.749–762. Available at: https://doi.org/10.2217/rme.10.58
Lou, Y.-R. et al., 2014. The Use of Nanofibrillar Cellulose Hydrogel As a Flexible Three-Dimensional Model to Culture Human Pluripotent Stem Cells. Stem Cells and Development, 23(4), pp.380–392. Available at: https://doi.org/10.1089/scd.2013.0314