T. Joki1, L. Ylä-Outinen1, L. Paasonen2, S. Narkilahti1
1 NeuroGroup, BioMediTech, University of Tampere, BioMediTech, Tampere, Finland
2 UPM-Kymmene Oyj, Helsinki, Finland
INTRODUCTION
Human stem cell based neuronal cultures are promising tools for studying e.g. disease mechanisms, drug response or developmental biology in vitro. However, traditional flat 2D cultures on top of a rigid surface fail to reproduce in vivo like brain complexity, like high cell density and connectivity with surrounding cell-cell and cell-extra cellular matrix (ECM) contacts. Due to these aspects, the 2D culture can lead to unnatural cell polarization on the flat culture. Different types of 3D cell cultures aim to overcome some of these limitations, by offering cells artificial extracellular matrix (ECM) and more in vivo mimicking environment.
The potential of nanofibrillar cellulose hydrogel (NFC, GrowDex®) for 3D culturing of various cell types has already been demonstrated (1, 2, 3). It mimics native soft tissue ECM in fiber size and in mechanical properties, thus providing cells a more in vivo like growth environment. GrowDex®-T is transparent, anionic version of NFC. In this study pre-differentiated human pluripotent stem cell derived neurons were cultured within GrowDex-T.
MATERIALS
- GrowDex-T 1% (Cat No. 200 103 005, UPM)
- 96-well plates, glass bottom (Cat No. P96G-1.5-5-F, MatTek)
- Human embryonic stem cell (hESC, Regea 08/023) derived neuronal cells
- 1:1 DMEM/F12: neurobasal media, supplemented with 2mM GlutaMax™,1xB27, 1xN2 and 25 U/mL penicillin/streptomycin (all from Thermo Fisher Scientific)
- Tryple Select (Cat No. 12563-011, Invitrogen)
- MAP2 antibody (Cat No. AB5622, Merck Millipore)
- β-Tubulin antibody (Cat No. T8660, Sigma-Aldrich)
METHODS
- hESC were pre-differentiated to neuronal cells using previously published method before encapsulation in GrowDex-T (4).
- Cells were seeded into GrowDex-T in concentration of 5x106 cells/mL.
- Tested GrowDex-T concentrations were 0.65, 0.45, and 0.30%.
- Two different hydogel volumes, 80 and 60 μL, were studied using a 96-well plate format. 150 μL media was added on top, and the cells were cultured for 14 days.
- The formation of the neuronal networks inside the hydrogel was evaluated by immunocytochemical staining against neuronal markers Microtubule-Associated Protein 2 (MAP-2) and β-Tubulin III (β-Tub), as previously described (5).
- Immunocytochemical samples were imaged with an Olympus IX51 inverted and Zeiss LSM 780-confocal microscope. Image processing was performed using Adobe Photoshop CS4, Huygens Essential, and ImageJ –softwares. Confocal data was visualised as shown in Fig. 1.
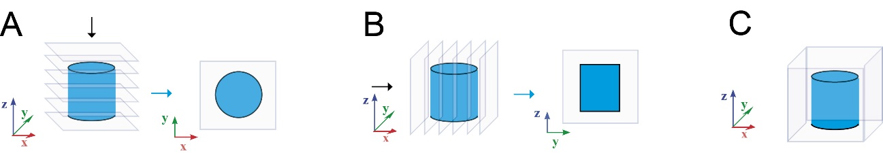
Figure 1. Image processing of the confocal stacks. Neuronal networks inside GrowDex-T were visualized using maximum intensity projections (A,B) and 3D rendering (C).
RESULTS
- GrowDex-T was stable 3D matrix
Sample preparation, cell plating, cell culture, and analysis were successful with GrowDex-T. The smaller GrowDex-T volume (60 µL) caused some loss of the material during washes in the immunocytochemical staining (Fig. 2). Overall, the samples were stable during cell culture and analysis. GrowDex-T was also highly suitable for phase contrast imaging due to optical transparency.
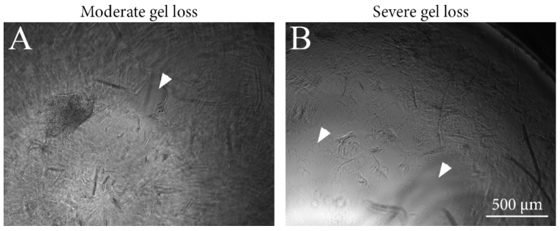
Figure 2. Images from representative GrowDex-T samples showing moderate and severe gel loss pointed by arrow heads. Images were taken after immunocytochemical staining and mounting. Scale bar is 500 μm.
- GrowDex-T volume affected cell growth
Neurite outgrowth was analysed from the immunocytochemically stained samples. Cultures were classified into three categories: 1) robust neurite outgrowth, 2) moderate neurite outgrowth, or 3) neural aggregates (no outgrowth), according to the amount of visible neurites (Fig. 3). Smaller hydrogel volume was more supportive of neurite outgrowth whereas the larger volume supported neural aggregates (Fig. 3B).
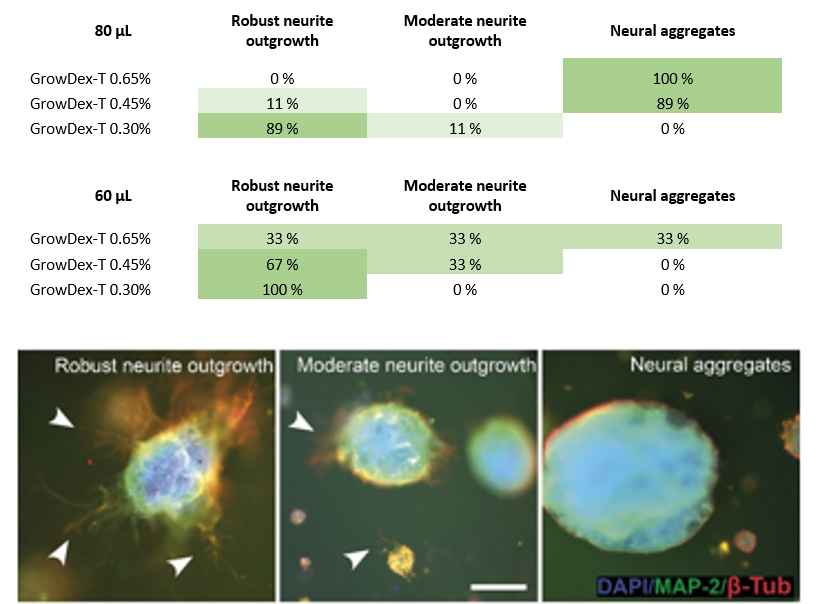
Figure 3. Evaluation of neurite outgrowth in different GrowDex-T volumes and concentrations (A), and examples of classification (B,C,D). Arrow heads show areas with neurite outgrowth. Scale bar is 200 μm.
- Robust neurite outgrowth in 3D
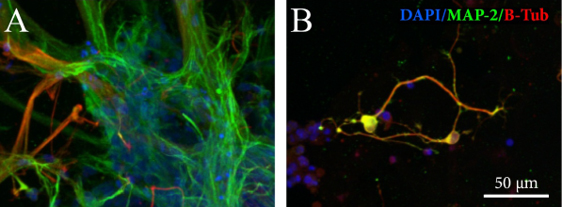
Neurite outgrowth was studied in more detail using confocal imaging. In 60 μL volume all GrowDex-T concentrations supported robust neurite outgrowth in all directions (Fig. 4). Lower GrowDex-T concentrations resulted in neurite outgrowth also from single cells.
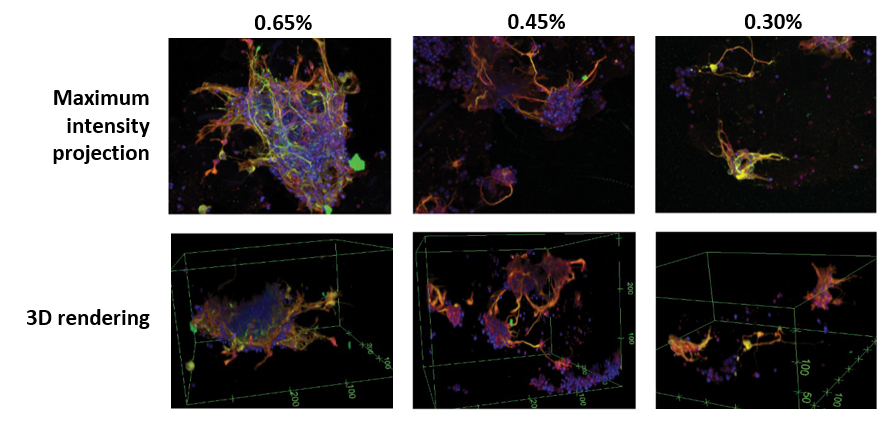
Figure 4. Maximum intensity projections and 3D rendering of confocal data (see Fig. 1 for the method). GrowDex-T concentrations used were 0.65%, 0.45%, and 0.30%. Volume in all samples was 60 μL. Markers in the images: DAPI (blue), MAP-2 (green), β-tubulin III (red).
CONCLUSIONS
Some differences in neurite outgrowth were observed between the three studied hydrogel concentrations and two volumes: neurite outgrowth was more robust in low GrowDex-T concentrations (0.30% and 0.45%) and in smaller volume (60 μL). Similar study was performed earlier with native GrowDex (6). The main difference between GrowDex-T and GrowDex results was in the growth of single cells and their neurite outgrowth; GrowDex supported neurite outgrowth mainly from cell aggregates, where GrowDex-T supported neurite growth also from single cells (Fig. 5). Depending on the application, both types of neurite outgrowth can be seen beneficial. For single neurons, it takes more time to form 3D networks. Thus, it can be hypothesized that prolonged culturing time would be beneficial to observe stronger network formation in these cases.
Based on the results it can be concluded that GrowDex-T offers a very good 3D growth environment for human neural cells, and it is a promising matrix for 3D neural in vitro models.
REFERENCES
1.Lou, Y.-R. et al., 2014. The Use of Nanofibrillar Cellulose Hydrogel As a Flexible Three-Dimensional Model to Culture Human Pluripotent Stem Cells. Stem Cells and Development, 23(4), pp.380–392.
2.Bhattacharya, M. et al., 2012. Nanofibrillar cellulose hydrogel promotes three-dimensional liver cell culture. Journal of Controlled Release, 164(3), pp.291–298.
3.Rinner, B et al., 2017. MUG-Mel2, a novel highly pigmented and well characterized NRAS mutated human melanoma cell line. Scientific Reports 7, 2098.
4.Lappalainen, R.S. et al., 2010. Similarly derived and cultured hESC lines show variation in their developmental potential towards neuronal cells in long-term culture. Regenerative medicine, 5(5), pp.749–762.
5.Koivisto, J.T. et al., 2017. Bioamine-crosslinked gellan gum hydrogel for neural tissue engineering. Biomedical Materials, pp.1–38.
6.UPM Biomedicals Application note AN015 “GrowDex® Supports Robust Human Neuronal Network Formation in vitro”