Long-term Culture of Human Biopsies in Nanofibrillar Cellulose Hydrogel
Johanna Niklander¹,², Lauri Paasonen³, Beate Rinner²
- Drug Research Program, Faculty of Pharmacy, University of Helsinki, Finland
- Division of Biomedical Research, Medical University of Graz, Austria
- UPM-Kymmene Corporation, UPM Biomedicals, Helsinki, Finland
INTRODUCTION
Most of the current in vitro cell models consist of one or two cell types, whereas to be physiologically relevant, the model should also consider the complex cellular interactions within tissue microenvironment. Animal-derived culture materials for 3D cell models are known to affect the diffusion environment of experiments, introducing xenofactor based bias to results, and affecting cell behavior via proteins and growth factors. Due to many challenges in reconstructing disease models in vitro, ex vivo samples have been gaining solid ground as experimental material. Intact biopsy pieces are easy and fast to process for cultivation including all cell populations of the studied biological target. When reliably validated for general use, the robustness of setting up a 3D biopsy culture model could significantly save time, costs, and resources in the industry.
In this study, our aim was to establish long-term tissue cultures from human biopsy pieces, and to evaluate the preservation of tissue-typical protein expressions. To qualify experimental culture model also for future clinical prospects, we utilized animal-free nanofibrillar cellulose (NFC, GrowDex®) hydrogel as culture scaffold. Wood-derived and biocompatible GrowDex is composed of nanocellulose fibrils having the fibril size and viscoelastic properties resembling extracellular matrix (ECM). To observe how GrowDex compares to commonly known animalderived culture scaffold, parallel biopsy cultures were done with mouse sarcoma basement membrane extract (BME) gel.
MATERIALS
- Healthy skin (HS) biopsy, juvenile foreskin (FRSK) biopsies via circumcision from patients younger than six years old, and glioblastoma (GBM) biopsy were received directly after surgery in DMEM solution
- DMEM (Cat No. 11880028, Gibco)
- RPMI-1640 (Cat No. 31870, Gibco) with 10% (v/v) FBS (Biochrom AG), 1mM L glutamine (Life Technologies) and 1x P/S Pen/Strep (Life Technologies)
- GrowDex (Cat No. 100103005, UPM Biomedicals)
- Matrigel Growth Factor Reduced (GFR) (Cat No. 356230, BD Biosciences)
- 96-well plates (Cat No. 140675, Nunc) and 6-well plates (Cat No 3628, Corning)
- Light microscope Olympus TH4-200
- HistoGel (Cat No. HG-4000-012, ThermoFisher)
- Paraffin processing instrument VIP Tissue Tek 5E (Sakura)
METHODS
Table 1. GrowDex and Matrigel culture scaffold components.
- Pre-calculated stock volumes of GrowDex and ice-cold Matrigel (Table 1.) were pipetted into 2 ml sterile Eppendorf tubes, where the tube receiving Matrigel was holding precalculated volume of ice-cold culture medium and was positioned in a frozen tube rack or in ice. Medium for GrowDex was pipetted directly into the hydrogel after the addition of GrowDex stock volume to tube. Both scaffolds were mixed with careful pipetting within the Eppendorf tubes.
- 50 µl of ready GrowDex and Matrigel culture scaffolds were added to 96-culture wells and placed into incubator for a minimum of 30 min (GrowDex) and 1h (Matrigel).
- Biopsies were moved into a 6-well plate holding enough warm DMEM to cover the tissue.
- Using sterile surgical scissors and tweezers, the biopsies were dissected into an average of 1.5-2 mm pieces that were inserted as flat tissue layers on top of the pre-incubated GrowDex and Matrigel scaffold layers.
- 50 µl of GrowDex or Matrigel culture scaffolds were added on top of the inserted biopsy pieces, and the assembled 3D culture plates were further incubated for a minimum of 30 min (GrowDex) and 45 min (Matrigel) in incubator.
- 130 µl of culture medium was carefully added on top of each culture well scaffold (Fig. 1). Additional liquid reservoir wells were found to be recommendable to avoid medium evaporation.
- Suspension cultures were done to acquire visual control condition for tissue viability and to observe the cell outgrowth rate.
- Culture medium on top of the scaffolds was changed every 3 days during the cultivation.
- Morphology development of cultivated biopsy pieces was observed with a light microscope on days 1, 6, 12, 18 and 27.
- Biopsy samples were collected on days 0, 3, 6, 12 and 28.
- Ex vivo biopsies in suspension were briefly rinsed in PBS(-) and incubated in 4% formalin at RT overnight, and then at 4 °C for 24h.
- GrowDex and Matrigel –cultivated samples were collected directly from the culture well scaffolds with a pipette tip, followed by brief PBS(-) rinse and the described formalin incubation.
- Fixed samples were stored in 70% EtOH at 4 °C before paraffinization. Formalin-preserved pieces were rinsed from EtOH remnants and encapsulated in HistoGel.
- Liquidified HistoGel was solidified into a parafilm-lined Eppendorf lid as a 30 µl layer. Prefixed and rinsed biopsy piece was placed on top of the gel. Another 30 µl layer of warm HistoGel was applied on top of the biopsy and solidified at RT.
- HistoGel-embedded samples were further incubated in 4% formalin for 2h before transferring to paraffin-embedding sample cassettes and paraffin processing equipment VIP Tissue Tek 5.
- Histological analyses were done in the Diagnostic and Research Institute of Pathology, Medical University of Graz. Biopsy samples were stained with hematoxylin-eosin (HE) and evaluated with antibodies characteristic for each tissue type (Table 2.).
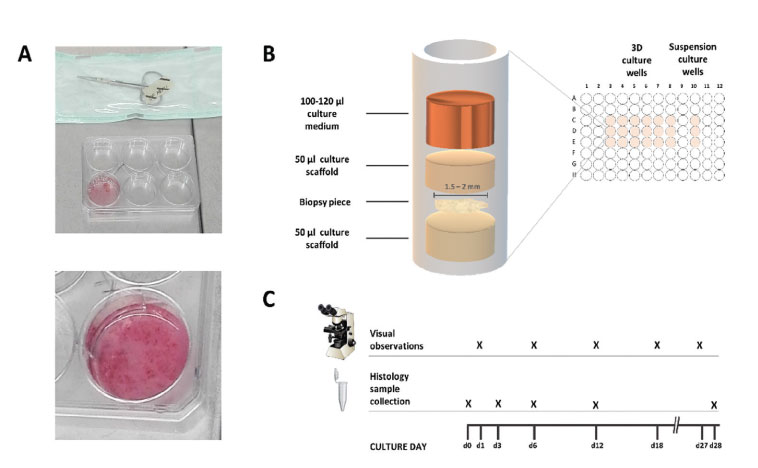
Figure 1. Experimental set up. Dissection of biopsies (A). Culturing of biopsy pieces in scaffolds (B). Biopsies were collected for histological analysis during biopsy dissection (d0) and from 3D cultures on days 3, 6, 12 and 28 (C).
Table 2. Antibodies used in immunohistochemistry analysis.
RESULTS
Healthy skin and foreskin biopsy cultures
Visual observations of HS and FRSK skin tissues revealed differing behaviors in vitro [2]. HS biopsies appeared relatively static during GrowDex cultivation, and GrowDex-cultivated FRSK tissues showed growth of tissue mass, development in the biopsy shape, and signs of protruding or degrading structures. When cultivated in Matrigel scaffolding, rapid cell spreading, tissue growth and biopsy unfolding were observed and the reductions in biopsy size correlated with rapid cell spreading. Histological analysis of HS biopsies indicated active maintenance of tissue integrity when cultivated in GrowDex. Also, analysis of FRSK biopsies showed epidermal tissues and additional dermal tissue. Matrigel-cultivated biopsy piece was lacking clear tissue layers and consisted of darker hematoxylin-stained biopsy tissue surrounded by pink eosin-stained protein growth [2].
Glioblastoma biopsy cultures
Visual observations of GBM biopsies showed protruding cells. GBM cultures showed variation in the development rates of individual culture wells and most prominent size reductions in cultivated tissue pieces. Typical morphology development during both GrowDex and Matrigel cultivation included partial degradation of original biopsy structures (GrowDex; Fig. 2A-D) and appearance of protruding cells (Fig. 2I-K). Extensive cell spreading in 3D Matrigel correlated with biopsy size reductions (Fig. 2E-H).
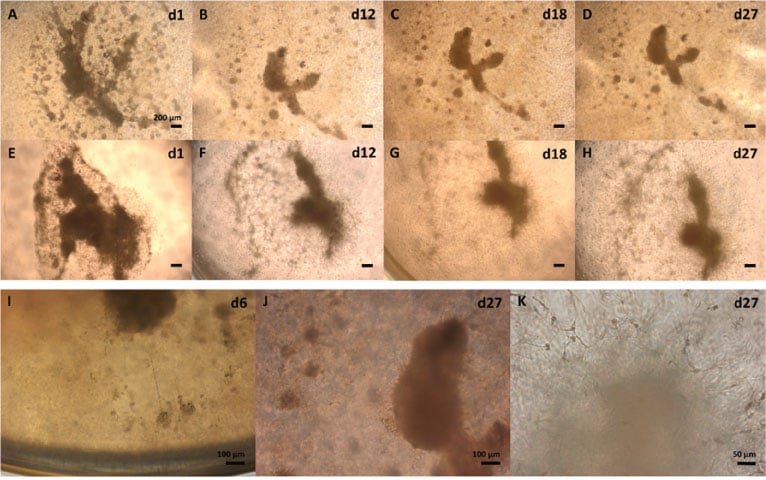
Figure 2. Morphology development of cultivated human glioblastoma multiforme (GBM) biopsy pieces in 0.7% GrowDex and 40% Matrigel.
HE staining of GBM biopsies revealed spindle shaped, highly pleomorphic tumor cells (Fig 3A, E, I). Antibody analysis revealed sustaining ex vivo protein expression profile in GrowDex and Matrigel culture conditions until day 28. Immunoreactivity to oligodendrocyte transcription factor 2 (Olig2) had evenly distributed nuclear stain from ex vivo (d0) to day 28 in vitro (Fig 3B, F, J). Similarly, calcium ion binding protein B (S100) detection remained stable from ex vivo to day 28 in vitro (Fig 3C, G, K). Neuroectodermal stem cell - intermediate filament VI (Nestin) was detected in cultivated day 28 samples (Fig 3D, H, L). Additionally, transmembrane ligand receptor NOTCH1 had peripheral location in GrowDex-cultivated samples, but NOTCH1 was not positive in day 28 sample cultured in Matrigel [2]. Immunoreactivity to microtubule-associated protein 2 (MAP2) showed peripheral staining in GrowDex-cultivated sample. Also astrocyte intermediate filamentspecific glial fibrillary acidic protein (GFAP) showed peripheral staining in GrowDex-cultivated samples on day 28, but staining was not observed in Matrigel-cultivated samples.
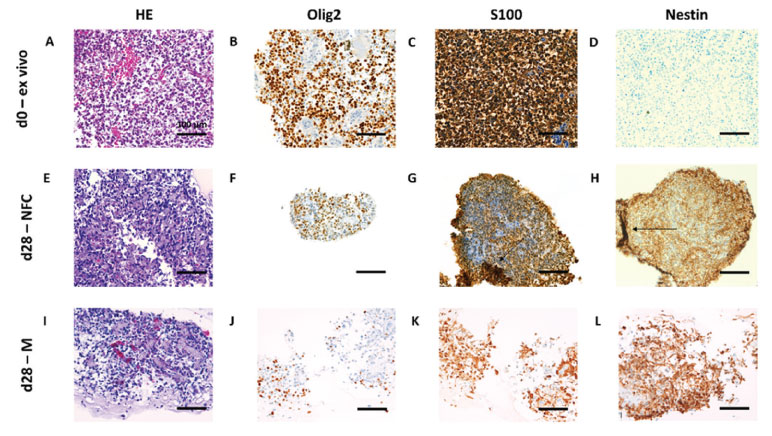
Figure 3. Histological hematoxylin-eosin (HE) tissue structure- and Olig2, S100 and Nestin epitope analysis from glioblastoma (GBM) ex vivo (day 0, 3A-D) and in vitro cultivated biopsy pieces (day 28) in 0.7% nanofibrillar cellulose (GrowDex, 3E-H) and 40% Matrigel (3I-L).
CONCLUSIONS
We report here the 3D culture assembly, optimized maintenance protocol and sample preservation methodology for GrowDex-cultivated biopsy pieces. The histopathological profiling indicated that biopsy pieces survive in long-term GrowDex cultivation and remain capable of sustaining their histological tissue identities. In addition to tumor modelling, we hypothesize this novel tissue culture model applicable to the modelling of regenerative and pathological processes that share many molecular features and regulatory mechanisms with cancers.
ACKNOWLEDGEMENTS
Authors thank Raili Koivuniemi, Alexander Stallinger, Florian Kleinegger, Silke Schrom, Bernadette Liegl-Atzwanger, Iris Zalaudek, Gord von Campe, Georg Singer, Johannes Haybaeck, and Marjo Yliperttula for their valuable input, as well as staff in the Core Facility Alternative Biomodels and Preclinical Imaging and Diagnostic and Research Institute of Pathology at the Medical University of Graz for their effort during the methodology development.
REFERENCES
- Niklander and Rinner (2021) Human Biopsies in Nanofibrillar Cellulose Hydrogel – A Novel Method for Long-term Tissue Culture. Drug Target Review, Issue #4 https://www.drugtargetreview.com/article/99931/human-biopsies-in-nanofibrillarcellulose-hydrogel-a-novel-method-for-long-term-tissue-culture/
- Niklander et al. (2021) Human Biopsies in Nanofibrillar Cellulose Hydrogel – A Novel Method for Long-term Tissue Culture. Biorxiv DOI: https://doi.org/10.1101/2021.11.22.466872