Ulla Saarela1, Jonathan Sheard2 and Seppo Vainio1
1. Laboratory of Development Biology, Organogenesis, Faculty of Biochemistry and Molecular Medicine, InfoTech Oulu, Kvantum Institute, University of Oulu
2. UPM Biomedicals, Helsinki, Finland
Introduction
Chronic kidney disease (CKD) is a syndrome linked with persistent changes and abnormalities in kidney structure and function. The underlying diseases which are most commonly associated with the development of CKD are diabetes mellitus and hypertension, and patients who develop CKD are at higher risks of cardiovascular disease [1]. Since it is difficult to restore kidney function following CKD, kidney transplantation is limited and dialysis cannot cure the disease, an alternative approach is needed [2].
Renal organoids mimic the structure and function of in vivo kidneys. They can be made from primary embryonic metanephric mesenchyme (MM) cells or using stem cells, such as human induced pluripotent stem cell (hiPSC) [3]. Renal organoids can be used for many applications, such as drug testing, disease modelling, studying kidney development and potentially they will be used for kidney function regeneration. When cultured in the lab, these primary cells are very sensitive and the nephrogenesis process can be negatively affected by many chemicals.
GrowDex® nanofibrillar cellulose (NFC) hydrogels are animal free and tunable extracellular matrices that support the culture of various cell types, including renal organoids. The NFC hydrogels are also sheer thinning and room temperature stable, which enable them to be used in 3D bioprinting applications.
In this study we examined whether MM derived renal organoids could be embedded in GrowDex, if the organoids were able to survive without negative effects, and whether it was possible to induce nephrogenesis.
The renal organoids were cultured using primary mouse embryonic kidney MM cells and chemically induced to undergo nephrogenesis. Pax2 and LTL (Lotus Tetragonolobus Lectin) were used to visualize and characterize the kidney and proximal tubules respectively.
GrowDex was the scaffold of choice for these cultures since it is simply composed of nanofibrillar cellulose and water providing a clean and well-defined matrix where the organoid can grow in a more natural 3-dimensional way, better mimicking physiological conditions.
 Kidney organoids
Kidney organoids
- Modeling kidney diseases
- Drud screening and nephrotoxicity test
- High thgoughput screening
- Regenerative therapy
Materials
- Primary mouse embryonic metanephric mesenchymal cells (MM cells)
- Timed E11.5 CD1 pregnant mice (Charles River)
- Complete media: DMEM-High glucose (Cat. No. 41965-039, Gibco), supplemented with 10% heat inactivated Fetal Bovine Serum (Cat. No. 10500-064, Gibco), 1% Penicillin/Streptomycin (Cat. No. P4333, Sigma)
- Dissociation buffer: 0.25% Pancreatin (Cat. No. P3292, Sigma) and 0.75% Trypsin (Cat. No. T4799. Sigma)
- Collagenase III (Cat. No. LS004182, Worthington)
- Physiological buffer: 137mM NaCl, 5.6mM KCl, 2.2mM CaCl2, 1.2mM MgCl2 x 6H2O, 2.5mM glucose, 10mM HEPES
- BIO ((2’Z,3’E)-6-Bromoindirubin-3’-oxime) (Cat. No. B1686, Sigma)
- Culture dishes (Cat. No. 627160 CellStar, Greiner Bio-One)
- Nuclepore Polycarbonate Track-etch membrane (Cat. No. 110608, Whatman)
- Metal grid
- GrowDex (Cat. No. 100 103 010, UPM Biomedicals)
- Paraformaldehyde, 4% (PFA) (Cat. No. 158127, Sigma)
- Glycine-PBS: 0.3M Glycine (Cat. No. 101196X, BDH Chemicals, VWR) in PBS
- Permeabilization and blocking buffer: Dulbecco's PBS (Cat. No. X0520-500, Biowest) with 20% goat serum (Cat. No. 16120064, Gibco), 10% BSA (Cat. No. B9001S, New England BioLabs), 1% Triton X-100 (Cat. No. T8787, Sigma)
- PBS (Cat. No. D1283, Sigma)
- Pax2 (1:200, Cat. No. 901001, Lot: B226007, BioLegend)
- Goat anti-Rabbit IgG (H+L) Highly Cross-Adsorbed Secondary Antibody, Alexa Fluor 546 (1:1000, Cat. No. A-11035, Invitrogen)
- LTL (Lotus Tetragonolobus Lectin Fluorescein) (1:30. Cat. No. FL-1321, VectorLab)
- Hoechst 33258 Staining Dye Solution (1:2000, Cat. No. ab228550, Abcam)
- ImmuMount (Cat. No. 9990402, Shandon, ThermoScientific)
Methods
- Preparation of the MM cells:
- Dissect embryonic kidney from the E11.5. mouse embryos.
- Treat the intact kidneys in dissociation buffer for 36 seconds and pipet into a glass dish with medium, neutralizing the dissociation buffer.
- Separate the mesenchyme from the ureteric bud using needles.
- Dissociate the mesenchyme for approximately 30 minutes in 40µl Collagenase III and 280µl Physiological buffer, pipetting the cells every 10 minutes. When the cells have been dissociated,add 500µl complete media to neutralize the Collagenase III.
- Wash the cell by centrifugation at 1380x g for 5 minutes and change the media gently. Repeat this wash step.
- Formation of renal organoids:
- Divide the MM cells into Eppendorf tubes containing 500µl of 10µM BIO in complete media.
- Centrifuge at 1380x g for 5 minutes.
- Incubate at 37°C (5% CO2) for 19 to 23 hours.
- Culture in GrowDex:
- Prepare Trowell culture dishes with a Nuclepore polycarbonate membrane on top of a metal grid in normal medium [4].
- Apply a layer (~250µl) of stock GrowDex (1.5%) on top of the membrane. For control samples no hydrogel was used.
- Using a pipette, place the renal organoids either on top of the Nuclepore polycarbonate membrane (control) or embedded inside the GrowDex and incubate for 5 days in 5% CO2 at 37°C.
- Fixing of the organoids:
- Place organoids in 4% PFA for 30 minutes. This can be done by placing the organoids into a new plate with 4% PFA within each well, or alternatively replacing growth media with 4% PFA.
- Following fixation wash the organoids in Glycine-PBS for 30 minutes.
- Remove the Glycine-PBS and add 1 x PBS.
- Continue for immunostaining or store in +4°C tightly sealed.
- Immunostaining of organoids (in 48-well plates)
- Remove 1 x PBS and add permeabilization and blocking buffer for 30 minutes.
- Remove the buffer, add primary antibodies diluted in PBS -/- then keep overnight in +4°C.
- Wash 6 times for 10 minutes in PBS -/-.
- Dilute the secondary antibody (AF488) in PBS and incubate for 1 hour in RT covered from light.
- Wash 3 times for 10 minutes in PBS -/-.
- Dilute Hoechst in PBS and incubate for 5 minutes in RT.
- Wash with PBS -/- and finally with H2O.
- Place in ImmuMount and image with confocal microscope (Zeiss LSM 780 laser scanning confocal microscope)
Results and discussion
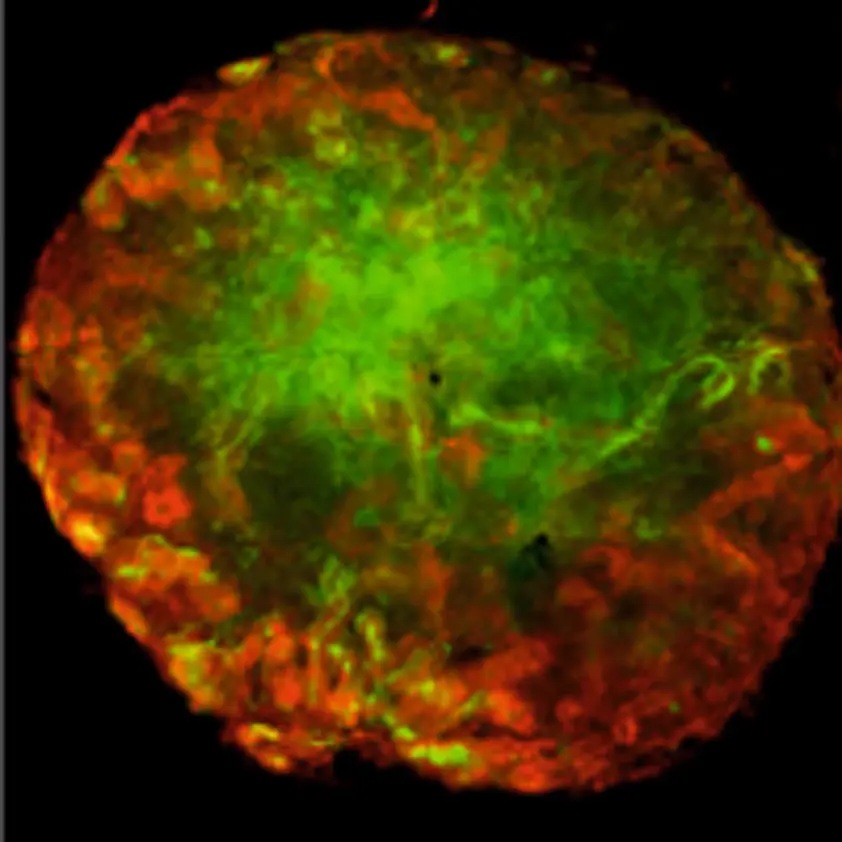
The primary MM based renal organoids were formed and then cultured either on top of a membrane without hydrogel in Trowell culture (Figure 1. A) or embedded in GrowDex (Figure 1. B). The organoids were grown for 5 days, followed by fixing and immunostaining for confocal imaging. Pax2 staining of the kidney tubules (Figure 2. red) shows that the nephrogenesis proceeds similarly to the organoids grown without hydrogel, whilst LTL staining (Figure 2. green) shows mature proximal tubules in both cases. Both organoids show numerous nephrogenesis. In GrowDex, the organoids grow in a more spherical form than in the control Trowell culture organoids, where the air pressure slightly flattens the organoid.
Figures
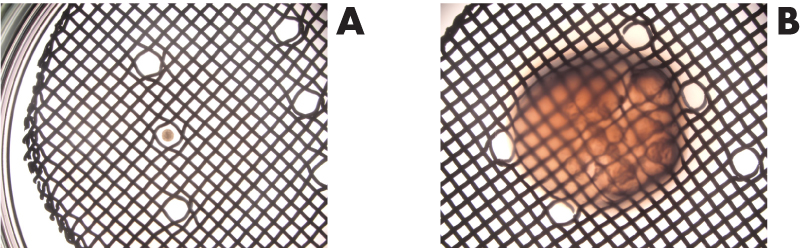
Figure 1. A) Trowell culture without hydrogel, organoid on top of the membrane and metal grid.
B) Trowell culture with organoid embedded in GrowDex on top of the membrane and metal grid.
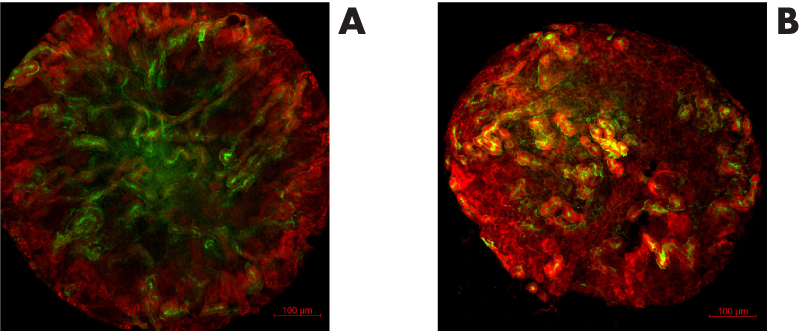
Figure 2. ICC staining of renal organoids grown for 5 days in Trowell culture in A) no hydrogel or
B) GrowDex. Organoids were stained with Pax2 (kidney tubules, red) and Lotus Tetragonolobus Lectin (LTL, proximal tubules, green). Scale bar 100µm.
Conclusions
The primary MM cells used in this experiment are highly sensitive to many chemicals and therefore nephrogenesis can be easily distorted. Here we tested the response to and behavior of the renal organoids made from primary MM cells when cultured in GrowDex.
The renal organoids cultured in GrowDex showed no signs of toxicity, and nephrogenesis proceeded as that seen with the control organoids. Immunostaining with Pax2 and LTL antibodies showed multiple developing nephrons and maturation of proximal tubules. Here we conclude that 1) GrowDex is not toxic for the MM cells, 2) nephrogenesis proceeds normally when renal organoids are cultured in GrowDex and 3) the renal organoids are more spherical when embedded in GrowDex than in the traditional Trowell cultures. These results suggest that GrowDex can be used for renal organoid culture and these hydrogels are also suitable matrices for other applications such as renal organoid bioprinting.
Advances in renal organoid generation from iPSCs, new culture techniques and innovative applications have and will continue to open up their potential use in many areas. Specifically, disease modelling and high throughput drug screening will provide better treatment strategies. Finally, the provision of renal organoids for clinical therapies toward kidney regeneration and restoration of kidney function will improve the lives of many patients suffering from CKD.
Acknowledgements: Biocenter Oulu Light Microscope Core, Veli-Pekka Ronkainen
Ethics: The animal care and experimental procedures in this study were done in accordance with Finnish national legislation on the use of laboratory animals, the European Convention for the protection of vertebrate animals used for experimental and other scientific purpose (ETS 123), and EU Directive 86/609/EEC.
"This work was supported by Academy of Finland grant 315030,
Wnt-mediated Kidney Tubule Induction – the Role of Secreted Extracellular Vesicles as Putative Morphogens."
References
- Romagnani P, Remuzzi G, Glassock R, Levin A, Jager KJ, Tonelli M, et al. Chronic kidney disease. Nature Reviews Disease Primers. 2017;3(1):17088. doi: 10.1038/nrdp.2017.88.
- Naganuma H, Nishinakamura R. From organoids to transplantable artificial kidneys. Transplant International. 2019;32(6):563-70. doi: https://doi.org/10.1111/tri.13404.
- Junttila S, Saarela U, Halt K, Manninen A, Pärssinen H, Lecca MR, et al. Functional Genetic Targeting of Embryonic Kidney Progenitor Cells Ex Vivo. Journal of the American Society of Nephrology. 2015;26(5):1126. doi: 10.1681/ASN.2013060584.
- Trowell OA. A modified technique for organ culture in vitro. Experimental cell research. 1954;6(1):246-8. doi: https://doi.org/10.1016/0014-4827(54)90169-X.