Teresa Mimler, Medical University of Vienna, Austria
Introduction
There are two main cell types in human blood vessels, endothelial cells and smooth muscle cells, which both are important for the blood vessel morphology and function. Better in vitro models are needed for the research of e.g. blood vessel formation, signalling between the cell types, and interactions with tissues. Traditional 2D cell culture is limited to cell monolayer, which does not resemble in vivo conditions. Novel 3D culture methods, such as hydrogels, provide better biological relevance and functionality.
In this study we demonstrate the separate 3D culture of two cell types present in blood vessels (smooth muscle cells and endothelial cells) in two different 3D matrices (GrowDex® and Matrigel™). GrowDex is a plant-derived nanofibrillar cellulose hydrogel, and Matrigel is an animal-derived basement membrane extract (BME) matrix.
Materials
- Primary human umbilical cord smooth muscle cells (SMC, self-isolated)
- Primary human umbilical cord vein endothelial cells (HUVEC, self-isolated)
- GrowDex 1.5% (Cat No. 100103002, UPM)
- Matrigel (Cat No. 354234, Corning)
- 96-well plate flat bottom (Cat No. 655 180, Greiner Bio-one)
- RPMI 1640 culture media supplemented with 10% FCS
- SMC culture media (Cat No. 200 0601, Provitro)
- EGM-2 media (Cat No. 200 0101, Provitro)
- 4% PFA supplemented with 0.8% TritonX-100, 5mM EDTA, and 1mM MgCl2
- Phalloidin Alexa Fluor 488 (Cat No. A12379, Invitrogen)
- CD31 (Cat No. MCA1746GA, BioRad)
- Alexa Fluor AF546 (Cat No. A11030, Molecular Probes)
- Hoechst 34580 (Cat No. HY-15560B, MCE)
Methods
1. Preparation of GrowDex
- GrowDex was diluted in a round bottom tube with RPMI 1640 media to a working concentration of 0.5%. Mixing was done by slowly pipetting up and down to achieve a homogenous solution without air bubbles.
2. Preparation of Matrigel
- Matrigel was left to thaw overnight in the fridge on ice. Also 96-well plate and pipette tips were cooled before use. Matrigel was diluted 1:6 with cold RPMI 1640 media and immediately put back on ice.
Methods contd.
3. Cell seeding on top of matrix
- 40 µL matrix was used per well on 96-well plate.
- Before adding 40 µL of matrix (GrowDex or Matrigel) per well, the wells were washed 2 times with RPMI 1640. The plate was placed in the incubator for 30 min before adding the cells.
- After the 30 minutes 10 000 cells per well in 200 µL culture media were seeded on top of the matrix and incubated at +37°C for 7 days.
4. Cell seeding embedded in matrix
- 100 µL matrix was used per well on 96-well plate.
- 10 µL cell suspension with 10 000 cells in culture media was prepared and mixed with 90 µL diluted matrix (GrowDex or Matrigel) per well.
- Cells embedded in matrix were dispensed into the well and put in the incubator for 30 min, 100µL culture media was added on top, and the plate was incubated at +37°C for 7 days.
5. Media change
- Culture media was changed after 3 days. 50 µL media was carefully removed and the same volume of fresh media was added.
6. Cell Fixation and staining protocol (1)
- Cells were fixed with 100 µL/well 4% PFA supplemented with 0.8% Triton X-100, 5 mM EDTA and 1 mM MgCl2 for 20 min at RT.
- Washing was performed 3 times with PBS-/-.
- Blocked with 100 µL/well 20% goat serum in PBS-/- for 1 h.
- SMC: Incubated with 50 µL/well Phalloidin-AF488 (2,5 µL/100 µL in PBS) for 3 h at RT.
- EC: Incubated with 50 µL/well CD31 overnight at RT.
- Washed 3 times with PBS-/-.
- EC: Incubated with secondary antibody AF546 with 100 µL/well for 3 h at RT and washed 3 times with PBS-/- afterwards.
- For nuclei staining incubated with 100 µL/well Hoechst 1:5000 in PBS-/- for 3 h at RT.
- Washed 3 times with PBS-/-.
- A final volume of 100 µL PBS-/- was then added and pictures taken using a confocal microscope.
Results and discussion
Both cell types, SMC and HUVEC, were cultured in 3D in GrowDex and Matrigel. Cultures were performed successfully with two different cell seeding methods: 1) cells were embedded in the matrices, and 2) cells were seeded on top of the matrices, respectively (Fig. 1).
Confocal microscopy after phalloidin-staining of actin filaments in SMCs suggested better cell viability and a spindle-shaped morphology with the cells seeded on top of GrowDex (Fig. 1, panels A and E), and the cell viability was similar to Matrigel cultures. The presence of endothelial cell marker CD31 in HUVEC cultures was also more pronounced with cell seeding on top of the matrices (Fig. 1, panels B, D, F, and H).
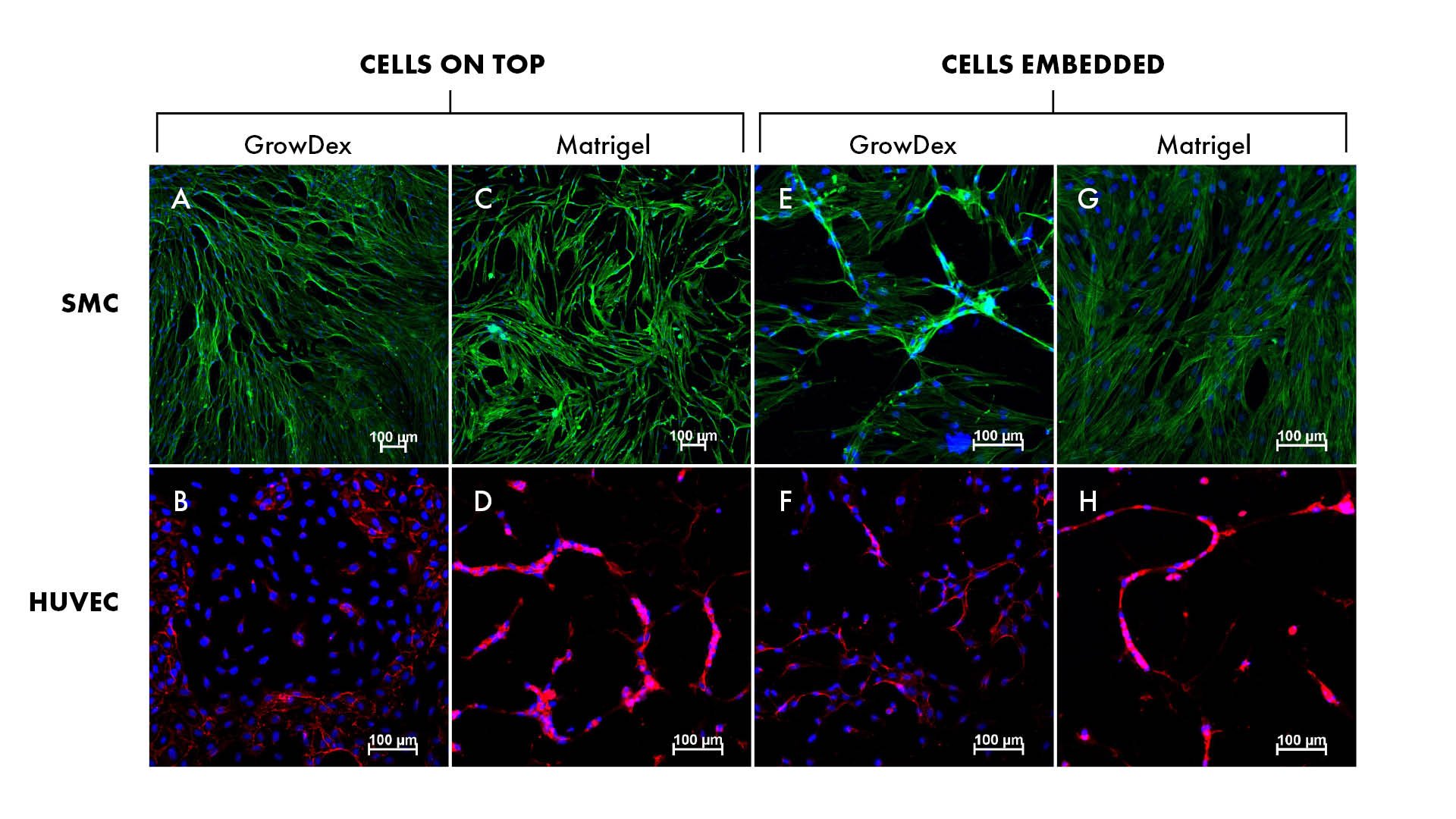
Figure 1. Smooth muscle cells (SMC, panels A, C, E and G) and umbilical cord vein endothelial cells (HUVEC, panels B, D, F and H) cultures in GrowDex and Matrigel.
Conclusions
Two cell types, SMC and EC, were successfully cultured in GrowDex and Matrigel respectively. Both cell types cultured in GrowDex resulted in good cell viability with cells being distributed evenly throughout the hydrogel. Cells seeded on top of GrowDex resulted in better cell viability and morphology, as analyzed by confocal microscopy. Confocal images of cells in GrowDex are of good quality, since the matrix is not auto-fluorescent and does not create any background interference.
Animal-derived matrices like Matrigel typically come with some challenges, such as variation between production batches, undefined composition and have been shown to influence gene expression in cells (2). Handling of BME matrices can also be tricky requiring low temperatures which can cause practical challenges, especially if scaling the assay to an automated platform.
Being a plant-derived hydrogel with well defined components and properties, GrowDex provides a clean matrix ensuring reproducible assay results. The hydrogel can be customized by addition of components to media and temperature stability and shear-thinning properties make the storage, handling and assay set-up easy. As a consequence of these properties, GrowDex is a suitable and preferred matrix for 3D culture of SMCs and ECs.
3D co-culture of the two cell types that are present in blood vessels and presented here would be beneficial in studying the interaction of cell types as an in vitro vascular model.
References
- Härmä V, Virtanen J, Mäkelä R, Happonen A, Mpindi JP, et al. (2010) A Comprehensive Panel of Three-Dimensional Models for Studies of Prostate Cancer Growth, Invasion and Drug Responses. PLOS ONE 5(5): e10431. https://doi.org/10.1371/journal.pone.0010431
- Corning (2018) Corning Matrigel Matrix. Retrieved from https://www.corning.com/emea/de/products/life-sciences/products/surfaces/matrigel-matrix.html