Juha Rantala, Ph.D., Misvik Oy, Finland, Lauri Paasonen, Ph.D., UPM Biomedicals, Finland
INTRODUCTION
Better in vitro cancer cell models are needed for e.g. cancer biology research and drug development. Traditional 2D cell cultures are being replaced with more functional and more physiologically relevant 3D cell models. Cancer cells derived from solid tumors or liquid biopsies, such pleural or ascites effusion, may require different culturing conditions. Anchorage-independency is a cellular feature facilitating the cells’ capacity to divide and function in the absence of surface to anchor with. Solid tissue cells may enter a programmed cell death (anoikis) when detached from their physical extracellular matrix (ECM), whereas metastatic cancer cells may bypass this mechanism in order to survive outside the primary tumor e.g. in bloodstream or effusions (1). Previously it has been demonstrated that nanofibrillar cellulose hydrogel, GrowDex®, offers a suitable 3D culture matrix for various cell types (2, 3, 4).
In this study we investigated the culture of two cancer cell lines in three conditions: 2D monolayer, in suspension, and in 3D using GrowDex. BT474 is a breast ductal carcinoma cell line derived from solid tumor, and COLO205 is a colorectal adenocarcinoma cell line derived from ascites metastasis.
MATERIALS
•GrowDex® (Cat No. 100103005, UPM Biomedicals)
•Cell culture medium
•RPMI-1640 medium (Cat No. BE12-7627, Lonza)
•2mM L-Glutamine (Cat No. 25030, Gibco)
•1% Penicillin-Streptomycin (Cat No. 15140, Gibco)
•10% Fetal Bovine Serum (Cat No. S181B-500, Biowest)
•Poly(2-hydroxyethyl methacrylate, pHEMA (Cat No. 18894, Polysciences Inc.)
•CellTiter-Glo (Cat No. 8462, Promega).
METHODS
•To minimize 2D cell growth on the well bottom, microwells for suspension and GrowDex cultures were coated with 1.2% pHEMA .
•For coating pHEMA was mixed with ethanol and 40 µL pipetted into the microwells. The coating was ready when all alcohol had evaporated leaving a thin hydrophobic film covering the plastic surface.
•Wells for conventional 2D monolayer culture were left untreated.
•Cultures in GrowDex were performed with 0.4% GrowDex concentration.
•Cell seeding density was 8000 cells / well in 100 µL with 50 µL medium on top.
•Cells were incubated in 5% CO2 , +37°C and the medium was refreshed every two or three days.
•Cell growth was monitored daily by microscopy using Motic AE2000 inverted microscope. Images were acquired on days 1, 3 and 6 using the 20x objective.
•Cell viability was quantified on days 1 and 6 using CellTiter-Glo® luminescent assay, whereby 50 μL assay reagent was pipetted directly into wells and samples incubated at +37°C for 1h. Luminescence was quantified using the Labrox plate reader (Labrox, Finland).
RESULTS AND DISCUSSION
BT474 cells formed loose clusters in suspension culture and round spheroids in GrowDex, while in monolayer culture the cells adhered tight to cell culture plastic and to each other forming seamless layers of cells (Fig. 1A). In GrowDex cultures the formation of spheroids occurred during the first day, resulting in symmetric, round and increasingly dense structures. Cell viability increased modestly in both monolayer and GrowDex cultures, reflecting the slow growing nature of the cell line (Fig. 1B). This was also evident from imaging both the pHEMA and GrowDex cultures, where the cellular structures did not increase significantly in size across the 6 day study.
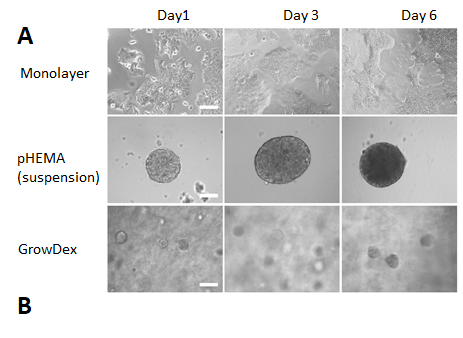
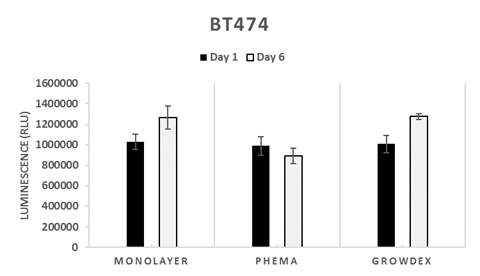
Figure 1. BT474 cells after 1, 3 and 6 days in monolayer, suspension (pHEMA) and GrowDex cultures (A). In GrowDex cells form multicellular tight spheroids (Light microscope, 20X objective, scalebar 100 μm). BT474 viability in GrowDex measured with CellTiterGlo shows 1.3-fold increase from Day 1 to Day 6 (B).
COLO205 cells showed a typical phenotype of suspension cells failing to differentiate into well-defined 3D structures (Fig. 2A). In the pHEMA coated wells the cells started to cluster indicating cell-cell interactions at day 6. In GrowDex the cells formed small clusters, but not round well-defined spheroids like BT474. Cell viability assay indicated that in monolayer culture there was a 3.4-fold increase from day 1 to day 6 (Fig. 2B). pHEMA culture displayed proliferation potential with a 6-fold increase from day 1 to day 6, in GrowDex the increase was 2.3-fold. The slower proliferation rate in GrowDex may be related to the fiber network affecting cell migration in the hydrogel.
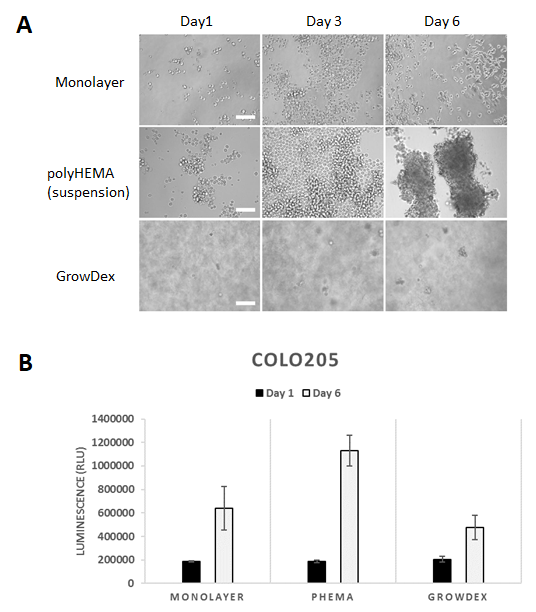
Figure 2. COLO205 cells after 1, 3 and 6 days in monolayer, suspension (pHEMA) and GrowDex cultures (A). In GrowDex the cells form small multicellular structures (Light microscope, 20X objective, scalebar 100 μm). COLO205 viability quantified with CellTiterGlo luminscence assay shows 2.3-fold increase from Day 1 to Day 6 in GrowDex (B).
CONCLUSIONS
Both cell lines BT474 and COLO205 were able to proliferate and form 3D multicellular structures in GrowDex. BT474 is derived from solid breast ductal carcinoma tumor, while COLO205 is derived from colorectal carcinoma ascites metastasis. BT474 showed the formation of round well-defined spheroids in GrowDex and loose cell clusters in suspension. COLO205 expressed the typical phenotype of suspension cells without organized 3D structure. In GrowDex small multicellular structures were observed.
The results suggest that GrowDex can offer a suitable matrix for cancer cells derived both from solid tumors and liquid biopsies. Nanofibrillar cellulose can be considered as a biologically inert material, but the anchorage-dependency does not seem be a limiting factor for 3D cell culture in GrowDex.
REFERENCES
1.Mori, S. et al. (2009). An Anchorage-Independent Cell Growth Signature Identifies Tumors with Metastatic Potential. Oncogene, 28(31), 2796–2805. http://doi.org/10.1038/onc.2009.139
2.Bhattacharya M. et al. (2012) Nanofibrillar cellulose hydrogel promotes three-dimensional liver cell culture, Journal of Controlled Release 2012; 164:3, pp. 291–298 https://doi.org/10.1016/j.jconrel.2012.06.039
3.Lou Y.-R. et al. (2014) The use of Nanofibrillar cellulose hydrogel as a flexible three-dimensional model to culture human pluripotent stem cells, Stem cell and development 2014; Vol. 23:4, pp. 380–392 https://doi.org/10.1089/scd.2013.0314
4.Rinner B. et al. (2017) MUG-Mel2, a novel highly pigmented and well characterized NRAS mutated human melanoma cell line. Scientific Reports 2017; 7, 2098 https://doi.org/10.1038/s41598-017-02197-y