GrowDex® product support
How to use GrowDex®
1. Calculate the needed volumes of hydrogel and medium
![]()
2. Pipette the medium
![]()
3. Before moving the cap from syringe, move gently the plunger back and forth to release it
![]()
4. Dispense the hydrogel to the medium
![]()
5. Swirl the mix gently with the pipette tip
![]()
6. Pipette gently up and down the mix for a minimum 90 seconds
![]()
7. Add the cells slowly and mix gently to avoid the bubbles
![]()
8. Dispense the cell mix to the wells
![]()
9. Add medium carefully on the top of the cell mix
![]()
GrowDex® calculator
Downloadable instructions for use
Material Safety Data Sheets
Frequently Asked Questions about GrowDex®
What are GrowDex hydrogels used for?
Applications are related to three-dimensional (3D) cell culture, including e.g. advanced in vitro cell models for drug development and toxicity, disease modelling, regenerative medicine research, and storage and transportation of biological samples.
Do GrowDex hydrogels include ECM proteins?
No. GrowDex hydrogels are clean and well-defined matrices consisting only of nanofibrillar cellulose and ultra-pure water.
What does GrowDex consist of?
Wood-derived nanofibrillar cellulose and ultra-pure water. There are no animal-derived components in GrowDex.
GrowDex is completely animal-free. BME matrices have components in them that I would like to have in the culture. Can I add them to GrowDex?
Yes, growth factors and proteins can be added to GrowDex. With GrowDex you have better control of the culture conditions adding what you need when you need it. Thus, you can easily repeat experiments with defined conditions and consistent results. GrowDex-A also enables the functionalization of the hydrogel with any biotinylated molecule.
Differences between the products
What are the main differences between different the GrowDex hydrogel products?
GrowDex is native nanofibrillar cellulose and the stock concentration is 1.5% (w/v).
GrowDex-T is anionic, transparent and the stock concentration is 1.0% (w/v).
GrowDex-A is anionic, transparent and it has avidins on the surface of the cellulose nanofibers, enabling the hydrogel functionalization with biotinylated compounds. Stock concentration is 1.0% (w/v).
Do GrowDex hydrogels need polymerization?
GrowDex hydrogels do not need to polymerize to form a gel. They are delivered in gel form and remain as a semi-solid hydrogel at all times, in temperatures from 0°C to 121°C.
How do you handle GrowDex hydrogels, as they are in hydrogel form all the time?
GrowDex hydrogels are each a shear-thinning material. It means that GrowDex is a semi-solid and highly viscous hydrogel at rest, but whenever a shear force is applied, it becomes more fluid temporarily. Immediately after the shear-force is stopped, GrowDex is a semi-solid hydrogel again. Shear force is generated e.g. during pipetting or injecting GrowDex. To learn more about the shear thinning properties of the GrowDex hydrogels, see our video “What does shear thinning mean?”
Please also see the ‘Instructions for Use’ -video at the top of this support page.
Can GrowDex hydrogels be handled with automated dispensing instruments e.g. for high throughput screening applications?
Yes. As GrowDex hydrogels are shear-thinning and temperature stable materials, they can be handled with automated systems in room temperature. These have been demonstrated with a variety of different dispensers, such as Biomek NXP, Gyger Certus Flex, Eppendorf EpMotion, Labsystems Echo 525, and ThermoFisher Multidrop.
Do GrowDex hydrogels have lot-to-lot variation? How do you control this?
No. Several physicochemical properties, microbiological purity, and standardized cell culture performance assays are analyzed for each lot. This way we can guarantee that cells behave the same way with each product lot we manufacture.
How should GrowDex hydrogels be stored and how long are they stable?
GrowDex and GrowDex-T can be stored at room temperature and have a shelf life of 12 months. Once opened, it is recommended that GrowDex hydrogels are stored in the fridge door for a maximum of 3 months to minimize the risk of microbial contamination.
The shelf-life of GrowDex-A and GrowDase is 6 months. Once opened, it is recommended that GrowDex-A hydrogels are stored in the fridge door for a maximum of 3 months.
How long is GrowDex stable after dilution?
When GrowDex is diluted with cell culture medium, GrowDex remains stable as long as the cell culture medium.
What cell types (cell lines, primary cells) can be cultured in GrowDex hydrogels?
More than 150 different cell types have been cultured in the GrowDex hydrogels. The adjustable stiffness enables the optimization of the culturing conditions for various cells. These include for example: stem cells, liver cells, cancer cells, neuronal cells, epithelial cells, and others. Several application notes and scientific publications are available in our Resource Center.
What is the optimal GrowDex hydrogel concentration and cell seeding density?
The optimal concentration and cell seeding density are dependent on the cell type. We recommend optimizing both for your assays. Please take a look at our application notes and GrowDex quickstart guide –video for further information. Additionally, please contact biomedicals.support@upm.com if you would like to enquire about a protocol for your application.
Are cells able to migrate in GrowDex hydrogels?
Yes. It has been demonstrated using live microscopy that cells, e.g. mesenchymal stem cells and fibroblasts are able to migrate in diluted GrowDex hydrogels. Since the nanocellulose fibres in GrowDex are not covalently cross-linked, the cells able to move through the hydrogels. Cells do not migrate in stock concentrations of GrowDex hydrogels due to the fibre density.
How do you analyze the cells in GrowDex hydrogels?
Which cell viability assay can be used with GrowDex hydrogels?
GrowDex hydrogels allow the free diffusion of chemicals and various viability assays can be used. The following viability assays have been successfully performed for the cells cultured in GrowDex hydrogels: CellTiterGlo 3D, CellTiterGlo, RealTimeGlo, AlamarBlue, PrestoBlue, LDH, LIVE/DEAD, WST, MTT, MTS, XTT.
Visit our Technical Protocols page to find common protocols which are routinely used for cells in GrowDex hydrogels.
Can the cells be stained and imaged in GrowDex hydrogels?
Yes. Different microscopy techniques, such as light microscopy, fluorescence microscopy and confocal microscopy have been successfully used to image cells in GrowDex hydrogels.
GrowDex hydrogels are not auto-fluorescent, and antibody stains are able diffuse through the hydrogels to the required sites.
Visit our Technical Protocols page to find common protocols which are routinely used for cells in GrowDex hydrogels.
How can cells be harvested from GrowDex for e.g. FACS analysis?
Cells can be liberated using GrowDase™ cellulse enzyme. GrowDase degrades nanocellulose fibres to soluble glucose and the gel structure breaks down for easy and gentle cell recovery. As neither human or animal cells contain cellulose GrowDase does not affect them, therefore the cell structures and surface morphology can be retained.
GrowDex® Troubleshooting
Here you can find frequently asked troubleshooting questions and answers related to the practical use of GrowDex hydrogel in 3D cell culture.
Handling and dilution issue
It is difficult to push the GrowDex hydrogels out of the syringe, it bursts out and how can I measure it more accurately?
Before opening the syringe screwcap, please pump the syringe plunger back and worth a few times. The seal on the syringe plunger may have gotten stuck to the syringe barrel, which may cause the sudden outburst of GrowDex hydrogel during dispensing.
Pipetting of GrowDex hydrogels seem difficult. Gel is stuck to the pipette tip and I cannot transfer it to the tube. Do you have any suggestions?
Pipetting of viscous materials like GrowDex hydrogels in their stock concentration needs to be done slowly. Currently, we provide GrowDex hydrogels in syringes to avoid the pipetting of hydrogel in stock concentrations. GrowDex can be dispensed directly from the syringe to the tube containing cell culture medium. Low-retention pipette tips should be used to avoid GrowDex sticking to the tip, e.g. Sarstedt low retention tips. Also, the use of wide-bore pipette tips, or cutting the pipette tip to make the hole larger makes the pipetting easier. Alternatively, positive-displacement pipettes work very well for pipetting GrowDex hydrogels.
Alternatively, GrowDex hydrogels can be measured by weighing. The density of GrowDex hydrogels equal the density of water.
I get air bubbles in my GrowDex hydrogel when diluting it with cell culture medium. Any advice how to prevent air bubble formation or how to remove the bubbles?
Aspirating and dispensing GrowDex hydrogels should be performed slowly to avoid air bubbles. Care needs to be taken that air is not introduced into the hydrogel. Specifically, the pipette should not be completely emptied when the pipette tip is inside the hydrogel. Serum in the culture medium increases the risk of getting air bubbles in the hydrogel.
If air bubbles are observed, they can be removed by leaving the diluted hydrogel in a fridge overnight, and bubbles will disappear. If bubbles do not disappear, then centrifuge the diluted hydrogel shortly at 200g for 5 mins. If phase separation occurs during centrifugation, please mix the hydrogel before use.
I tried to incubate the (stock) GrowDex hydrogels for a longer time at 37C but did not observe gel formation - it rather remained in the initial viscous and fluid state. This contrasts with the BME matrices that we use routinely. Is this expected?
Yes, it is expected that GrowDex hydrogels do not 'gel' or ‘cross-link’ because of temperature. It is stable in temperatures between 0-121°C, and it is delivered as a ready-to-use hydrogel. It consists of water and cellulose nanofibers that are not covalently linked together, but physically tangled together. Due to the shear-thinning property of the material, the hydrogel starts to flow when force is applied (e.g. pipetting or injecting), and the viscosity is retained when force is stopped.
If you would like to know more about the shear-thinning properties of the hydrogel, please see this video.
Gel Clumps
After diluting the GrowDex hydrogels, I see gel clumps in the tube. Is this normal and is there any advice how to avoid the clumps?
Clumps in the hydrogel are likely due to insufficient mixing. It is recommended to mix by pipetting up and down for a minimum of 90 seconds. If there are any visible clumps remaining after 90 sec mixing, the mixing should be continued until the hydrogel is homogenous.
I have plated the cells in GrowDex hydrogels to a 6-well plate. After overnight incubation at 37°C, there are gel clumps in the well.
We recommend using smaller well plates, such as 96-well plates for GrowDex hydrogel cultures. The smaller well provides more wall support for the hydrogel, and it remains more stable. The lack of adequate wall support in larger wells may cause the clumping of the hydrogel leading to a non-homogenous matrix. Please make sure also that the mixing was done properly before plating the hydrogel to the well plate. GrowDex hydrogels should be mixed by pipetting up and down for a minimum of 90 seconds.
Cells on the bottom of the well
The cells were embedded in GrowDex hydrogels, but after 24h incubation they were on the bottom of the well. How to avoid the 2D culture of the cells on the well bottom?
GrowDex hydrogels provide the cells with physical support to remain in 3D. The cells do not sink to the well bottom by gravity, but they are able to migrate through the GrowDex hydrogels.
If adherent cells are embedded in GrowDex hydrogels in a normal tissue culture treated cell-culture plate, they may migrate to the bottom and adhere. Therefore low-attachment and ultralow-attachment microplates are recommended for adherent cells to prevent the 2D cell growth on the bottom of the well.
Not all cells need the low-attachment plates. For example, pluripotent stem cells and A549 lung cancer cells have been cultured on standard cell culture treated plates (Nunc Microwell #167008) for PSC, and Greiner #655180 for A549). It is dependent on the cell type, if low-attachment plates are needed. For adherent cells such as HepG2, e.g. Corning Costar flat bottom ultra-low attachment (ULA) plates, and BRANDplates® inertGrade plates work well.
It is also possible to pre-coat the standard cell culture treated plates with a layer of 0.4% poly-HEMA to prevent the cells adhering to form a 2D culture.
It may be also possible that if the GrowDex hydrogels were not mixed thoroughly before embedding the cells, the cells may have sunk to the bottom of the well via the liquid channels which may form in the GrowDex hydrogel. To avoid this, careful and thorough mixing of GrowDex hydrogels should be done to have a homogenous culture matrix.
Changing culture media
Do you have any hints and tips for handling GrowDex during medium change? I want to avoid disturbing the cell-hydrogel when replacing the medium. A: Please see our ‘GrowDex 3D cell culture’ video showing the whole assay protocol including the culture medium change.
The plate can be tilted to an angle and medium removed from the top layer, above the layer of GrowDex. Applying the fresh culture medium on top of GrowDex should be done carefully against the well wall. If you are working with low concentrations of GrowDex (<0.5%), centrifuge the plate at 200g for 5 min before medium exchange to pack the hydrogel slightly to the bottom of the well. Additionally, to reduce the risk of accidentally removing the hydrogel during medium removal, it is recommended to replace only 50-80% of the medium.
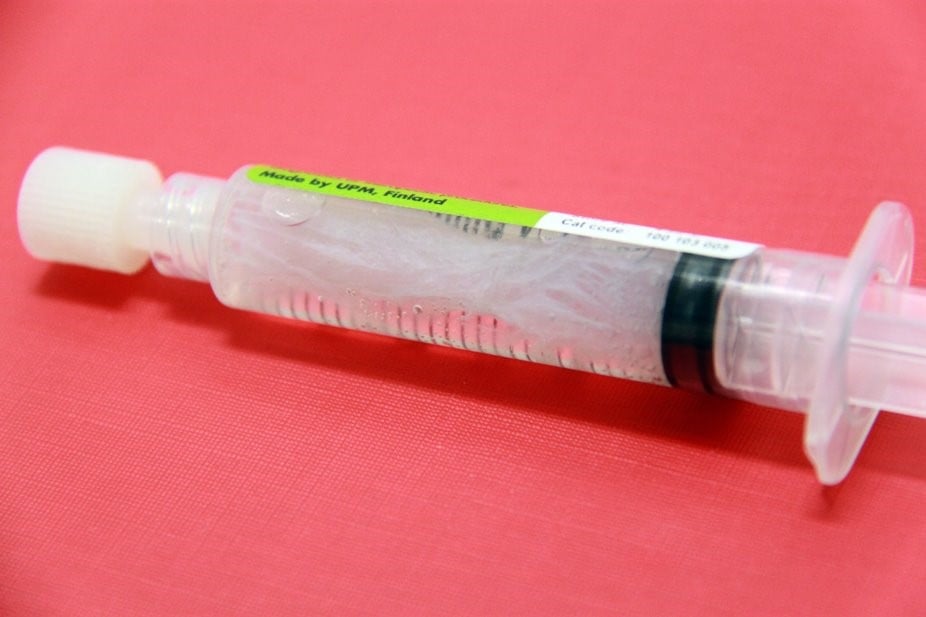
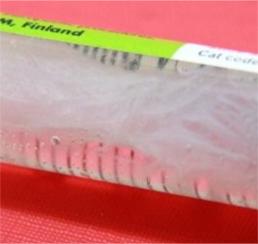
The water separates from the gel
GrowDex hydrogel in the syringe does not appear to be homogenous. It seems that water has separated from the hydrogel, see the image below. Is this normal and can I mix the water back to the hydrogel?
No, it is not normal, and the product should not be used. Water should not appear to be separated from GrowDex. It is likely that the product has been frozen at some point. During the freezing, the water separates from the nanofibrillar cellulose and it cannot be mixed back to the hydrogel anymore. Please do not use the product. GrowDex hydrogels should always be stored above 4°C.
Couldn't find what you were looking for?
Let us know how we can help by filling in the form below. The fields marked with an asterisk are required and please write briefly what your matter concerns, so we know who is the right person to contact you.