Comparative study of (immuno)-staining and confocal imaging of organoids in Matrigel®, GrowDex® and GrowDex®-T
André F. Maia1,2, Marta A. da Silva1, Jonathan Sheard3, Tomi Saviluoto3
1 i3S—Institute for Research and Innovation in Health, University of Porto, 4200-135 Porto, Portugal
2 IBMC—Institute of Molecular and Cell Biology, University of Porto, 4200-135 Porto, Portugal
3 UPM Biomedicals, Biomedicum 2U, Tukholmankatu 8, 00290 Helsinki, Finland
INTRODUCTION
Immunostaining multiple organoid samples can be a labour-intensive and time-consuming process, especially when multiple centrifugation steps are required for reagent exchange during fixation, immunofluorescence labelling, and washing. Each of these steps introduces the potential for sample loss, variability and mechanical stress, which may compromise organoids integrity. This challenge is further compounded when working with organoids grown under matrix-free conditions, such as those cultured in U-bottom ultra-low attachment (ULA) plates, spinner flasks or bioreactors. In these systems, organoids remain in constant motion, making it difficult to ensure uniform staining while also increasing the risk of sample aggregation or loss during processing.
Beyond the staining process itself, high-throughput confocal imaging of organoids presents additional technical challenges. Immobilization of these organoids within a matrix is therefore ideal or even necessary to prevent movement during imaging, ensuring consistent sample positioning across multiple fields of view. This is particularly important when using automated high-content screening platforms, where precise spatial registration is required for sequential image acquisition. Many high-content screening confocal microscopes are equipped with pre-screening capabilities, allowing for the initial acquisition of low-magnification images to identify and map organoid locations, followed by high-magnification imaging with multiple z-stacks to capture detailed three-dimensional structures. However, without a suitable immobilization strategy, organoids displacement between imaging steps can lead to inconsistencies in data collection and hinder automated analysis.
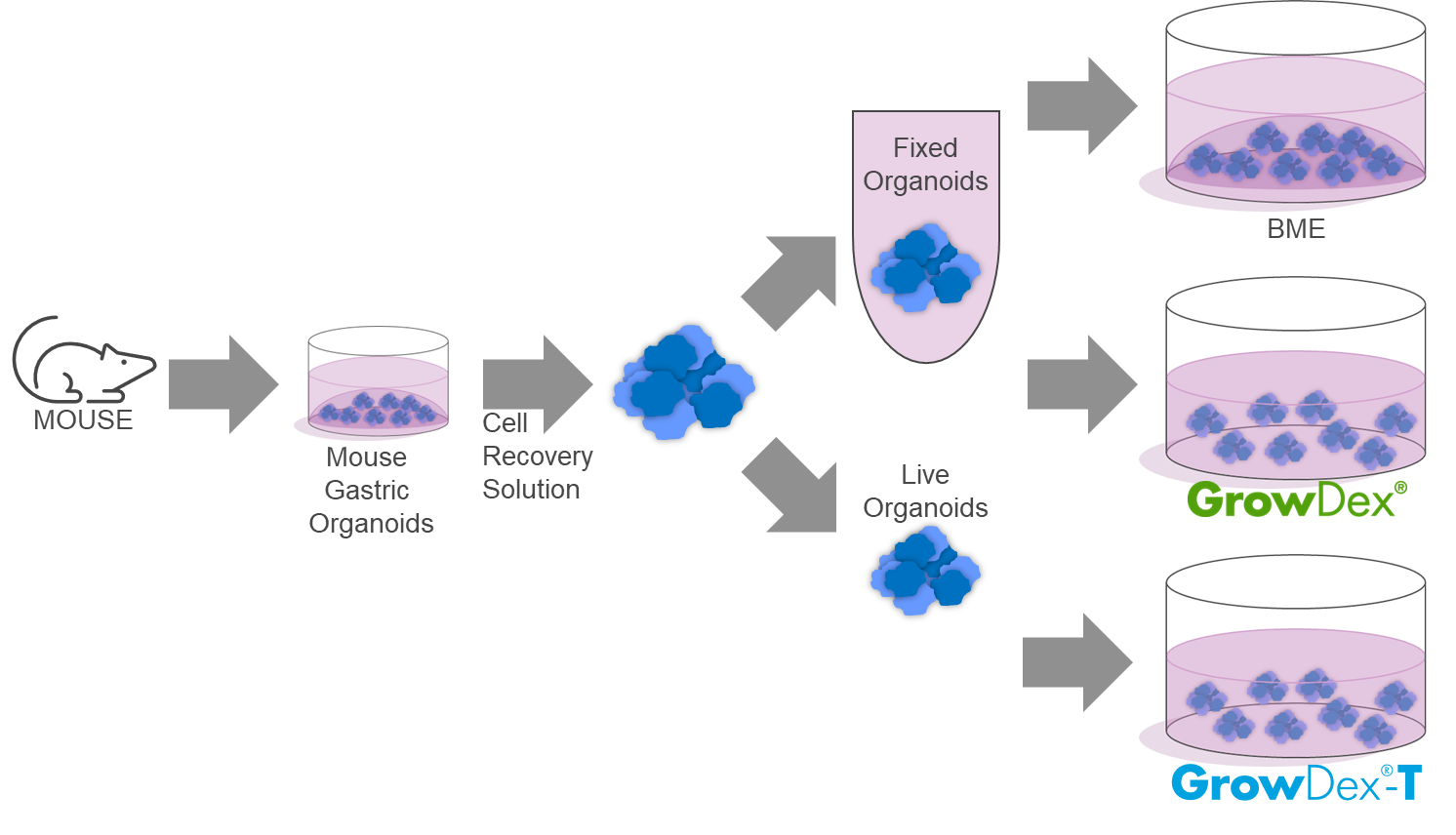
In this study, we utilized mouse gastric organoids cultured in Matrigel® as a model system. Following recovery, these organoids were re-embedded in Matrigel®, GrowDex®, and GrowDex®-T to evaluate their suitability for fixation, (immuno)staining, immobilization and high-throughput confocal imaging.
Thus, the objectives of this comparative study were to:
1. Investigate the impact of each matrix on the fixation process.
2. Assess matrix transparency for brightfield imaging.
3. Evaluate the efficiency of staining and immunofluorescence of organoids within different matrices.
4. Compare fluorescence confocal imaging performance across the tested matrices.
MATERIALS
- Murine gastric organoids isolated from stomachs of healthy surplus mice, according to Bartfeld, S. et al [1]
- Optically clear 96-well flat bottom microplate (PhenoplateTM 96, Cat No 6055300, Revvity, USA)
- Matrigel® (Cat No 356231, Corning®, USA)
- Cell Recovery Solution (Cat No 354253, Corning®, USA)
- GrowDex®, 1.5% (Cat No 100 103 005, UPM Biomedicals, Finland)
- GrowDex®-T, 1.0% (Cat No 200 103 005, UPM Biomedicals, Finland)
- Immunocytochemistry staining reagents:
- Primary antibodies: Ki67 1:100 (Cat No’s Ab15580, Abcam, UK) and Phalloidin-647 1:1000 (U0298, Tebubio, France).
- Secondary antibodies: Alexa FluorTM 488 at 2 µg/ml (Cat No A-11008, Thermo Fisher Scientific, USA)
- PhenoVue Hoechst 33342 (Cat No. CP71, Revvity, USA)
- Paraformaldehyde 20% Aqueous Solution (Cat No 15713, Electron Microscopy Sciences, USA)
- GibcoTM DPBS (Ca No 14190, Thermo Fisher Scientific, USA)
- TritonTM X-100 (Cat No. 9002-93-1, Merck, Germany)
- DMSO (Cat No. 0231, avantor, EUA)
- BSA (Cat No. A3311, Merck, Germany)
- FBS (Cat No. 12103C, Merck, Germany)
- Opera PhenixTM Plus high-content screening system, (Revvity, USA)
- Harmony Software 5.2.3 (Revvity, USA)
- ImageJ Software ver. 2.0.0-rc-69/1.52p. (National Institutes of Health, Bethesda, MA, USA)
METHODS
1. Murine gastric organoids were isolated from the stomachs of healthy surplus mice, following the protocol of Bartfeld et al. [1]. Briefly, murine stomachs were opened, washed, and cleared of veins and muscle tissue. The remaining tissues were minced and incubated with EDTA in chelation buffer. Stomach glands were mechanically isolated, centrifuged, and then seeded into Matrigel in 24-well plates for gastric organoid culture. The culture medium was added and changed every other day.
2. Gastric organoids at various stages of development were recovered from the Matrigel using the Cell Recovery Solution, following the manufacturer’s instructions.
a. A set of recovered organoids were fixed with 4% PFA/DPBS for 30 minutes at room temperature in a test tube. Upon fixation organoids were resuspended in DPBS prior to embedding in Matrigel, GrowDex or GrowDex-T.
b. The other set of organoids were embedded without fixation and fixed after embedding in the different matrices.
3. GrowDex (1.5% w/v) and GrowDex-T (1.0% w/v) were diluted, firstly with DPBS for a stock working solution of 0.8%. To achieve a homogenous mixture, GrowDex and GrowDex-T were mixed with DPBS by first swirling the pipette tip (cut tip) along the wall of the tube and then by pipetting up and down with a non-cut tip for a minimum of 90 seconds until a homogenous solution has been achieved by visual inspection. Air bubble formation was avoided by keeping the pipette tip submerged in the solution throughout the mixing process.
4. Embedding the organoids.
a. The pre-fixed organoids were mixed with Matrigel (100%) or mixed in a 1:1 ratio with pre-diluted working stock GrowDex or GrowDex-T (0.8%) to achieve a final seeding concentration of 0.4% GrowDex or GrowDex-T.
b. Similarly, the non-fixed organoids were mixed with the same matrices at the same ratios mentioned above.
5. Then, 100mL of the mixture containing organoids and matrix was transferred, with a cut tip, to each well of a Phenoplate 96.
6. Wells containing non-fixed organoids were incubated with 100mL 4% PFA/DPBS for 30 minutes at room temperature. After fixation, the 4% PFA/DPBS was removed and DPBS was added.
7. The following (immuno)-staining procedure was performed for both sets of organoids:
a. Permeabilization and blocking was performed with a PBSTD solution (DPBS, 0.3% Triton X-100, 1% DMSO, 1% BSA) for 60 minutes at room temperature.
b. Primary antibodies (anti-Ki67, Phalloidin-594) solution was prepared in a PBSTD-FBS solution (PBSTD, 5% FBS) and incubated overnight at 4°C. FBS should be freshly added to the PBSTD solution.
c. Washes were done in a PBS-BSA solution (DPBS, 1% BSA), 4 times for 5 minutes each.
d. Secondary antibody (anti-rabbit 488) and PhenoVue Hoechst solution was prepared in PBSTD-FBS and incubated for 3 hours at room temperature.
e. Washes were done in a PBS-BSA solution (DPBS, 1% BSA), 3 times for 5 minutes each. Followed by an extra wash with DPBS.
8. Image acquisition was done with an Opera Phenix Plus system, in confocal mode, using a Zeiss Plan-NEOFLUAR 10x/0,3 objective spanning 210mm with a 10mm z-step.
9. Image projection and rendering was performed with Harmony software. Image preparation for publication was performed with ImageJ.
RESULTS
- Evaluation of transparency for brightfield microscopy imaging
Gastric organoids at different developmental stages were recovered from Matrigel and embedded in either Matrigel, GrowDex, or GrowDex-T. One set of organoids were fixed with PFA before embedding, while another set of live organoids were embedded and then fixed. Brightfield imaging revealed no differences in transparency between the matrices, regardless of whether the organoids were pre-fixed or not. As expected, some cellulose fibres were visible in GrowDex but not in GrowDex-T, which is transparent similarly to Matrigel. Notably, organoids embedded in Matrigel appeared more crowded due to the confined space provided by the dome, whereas in GrowDex, they were more evenly distributed throughout the well.
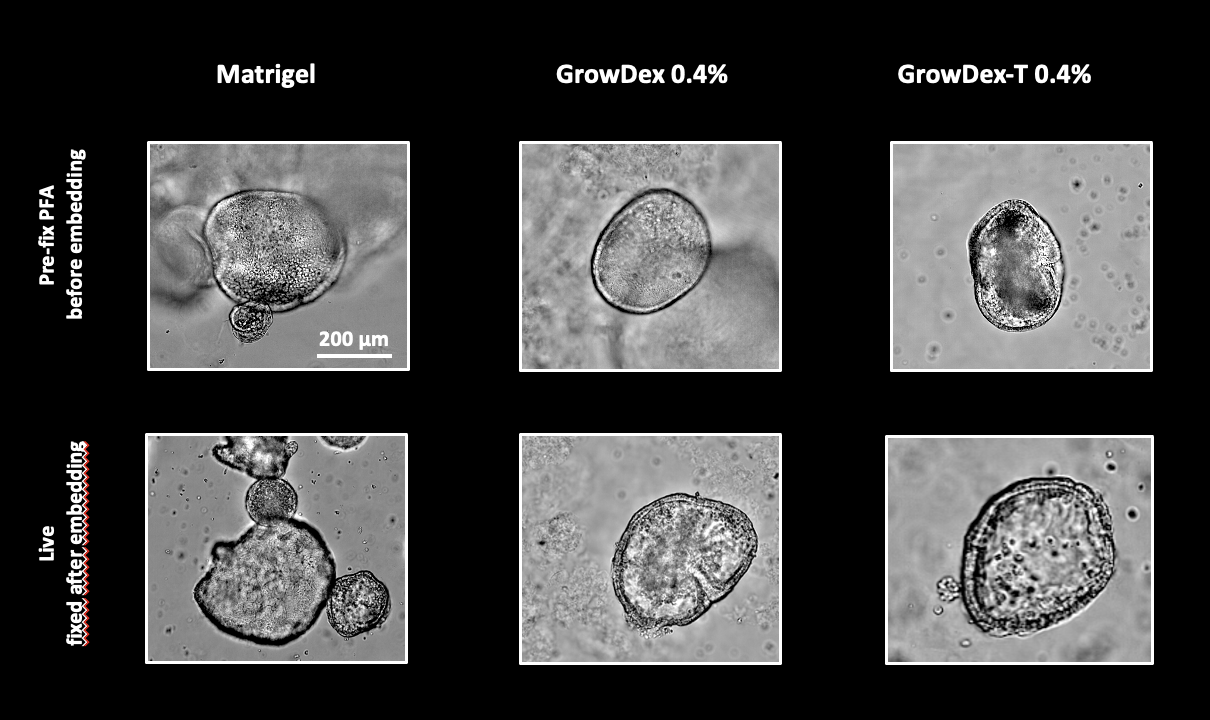
2. Evaluation of staining and immunofluorescence for confocal imaging
The same gastric organoids underwent an immunofluorescence procedure using Phalloidin conjugated with a 647 dye and anti-Ki67, followed by a secondary antibody with a 488 dye. Simultaneously, the high-content staining tool PhenoVue Hoechst was applied.
Image acquisition and reconstruction were performed using the high-throughput screening system Opera Phenix Plus, along with image analysis software. The results showed no differences in immunofluorescence or staining efficacy between the tested matrices, regardless of whether the organoids were pre-fixed or fixed upon embedding. GrowDex and GrowDex-T exhibited a performance comparable to Matrigel.
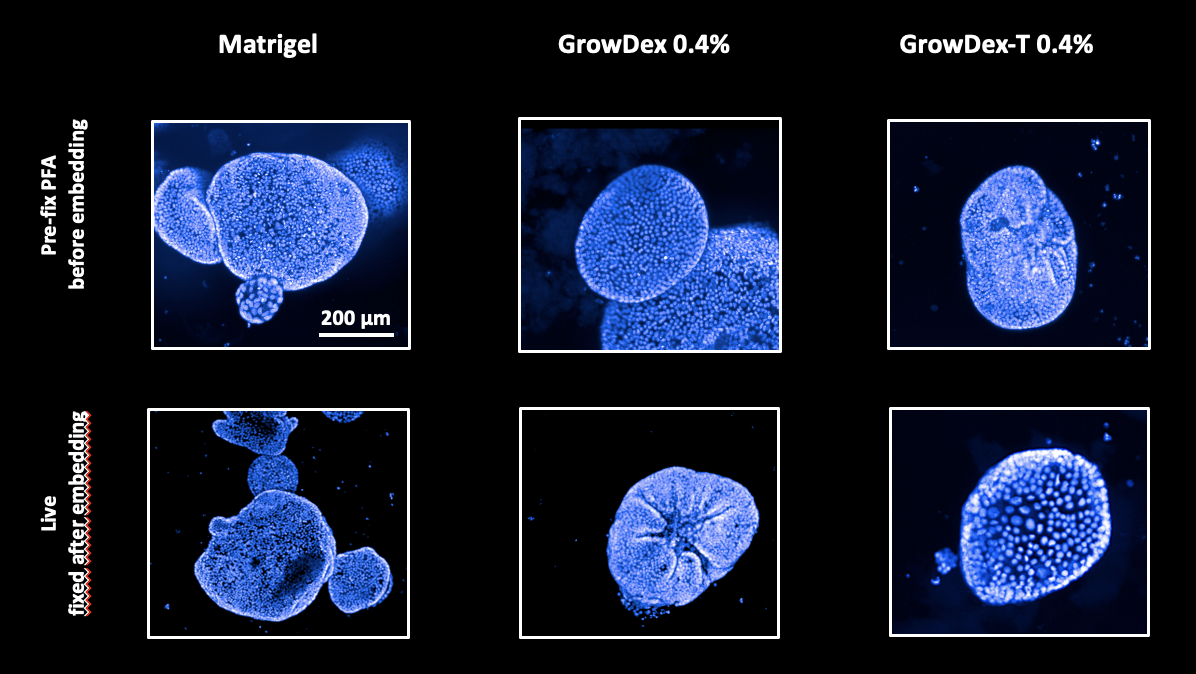

CONCLUSION
This study demonstrated that GrowDex® and GrowDex®-T are effective alternatives to Matrigel® for the embedding, fixation, staining, and high-throughput imaging of gastric organoids. Both hydrogels are easy to handle at room temperature and support the efficient embedding and dispensing of organoids into microplates, offering a convenient and reproducible method for immobilization at a concentration of 0.4%.
Importantly, organoids embedded in GrowDex® or GrowDex®-T performed comparably to those in Matrigel®, with excellent transparency for brightfield imaging and strong staining and immunofluorescence results. Notably, there was no significant difference in fluorescence intensity or matrix transparency, regardless of whether organoids were pre-fixed or fixed post-embedding, highlighting the versatility of GrowDex® and GrowDex®-T in maintaining sample integrity during the staining process.
The ability to perform experiments in an animal-free, inert hydrogel environment makes GrowDex® and GrowDex®-T valuable tools for 3D cell culture applications. These matrices not only provide stable support for organoid growth and immobilization but also allow for seamless integration with high-content screening systems. The findings from this study suggest that GrowDex® and GrowDex®-T offer a practical, scalable solution for high-throughput screening assays, enabling the detailed analysis of organoid biology with minimal sample manipulation and maximum experimental consistency.
The use of these matrices can significantly enhance the throughput and reliability of imaging-based screening platforms, facilitating large-scale analyses of organoid behaviour in response to different treatments or genetic modifications.
In summary:
- GrowDex and GrowDex-T are easy to handle at room temperature.
- Organoids can be easily embedded in GrowDex or GrowDex-T and dispensed into microplates.
- Good immobilization of organoids in GrowDex or GrowDex-T at 0.4%.
- GrowDex or GrowDex-T enables further experiments to be performed in an animal-free, inert hydrogel.
- Good transparency for brightfield imaging across all tested matrices.
- Suitable for staining with high-content tools and immunofluorescence.
- No difference in transparency or fluorescence intensity, regardless of whether organoids are pre-fixed before embedding or fixed afterward.
REFERENCES
- Bartfeld S, Bayram T, van de Wetering M, Huch M, Begthel H, Kujala P, Vries R, Peters PJ, Clevers H. (2015) “In vitro expansion of human gastric epithelial stem cells and their responses to bacterial infection.” Gastroenterology. 148(1):126-136.e6. doi: 10.1053/j.gastro.2014.09.042.
Read: High-quality nanofibrillar cellulose for biomedical use