Our OEM solutions for Flow Cytometry
Nanofibrillar cellulose and cellulase enzyme serve as a comprehensive solution for flow cytometry applications; convenient for cell culturing both before analysis & after cell sorting.
Our raw material provides an environment for cells that closely mimics the structure of extracellular matrix, allowing cells to interact & grow in similar manner than in human body. This enables more accurate and reliable data for flow cytometry analysis. In addition, nanofibrillar cellulose acts as an excellent scaffold for cells for further characterization and research after cell sorting.
1. Increased accuracy and relevance of results with 3D grown cells
Be a forerunner in providing 3D cell culture solutions that improve the quality of data obtained with flow cytometers.
To ensure scientific relevance of results from flow cytometry analysis, it is important to grow cells in habitats that mimic the human body as closely as possible. Although growing cells in a monolayer is a common practice, cells grown in 2D lack the physiological relevance of cells growing in human body. Therefore, the accuracy of the results can be questionable. To improve the physiological relevance, nanofibrillar cellulose acts as a scaffold for cells, allowing them to grow in three-dimensions closely mimicking the structure of cells growing in their natural habitat.
Although, with current flow cytometry technologies, 3D grown organoids and spheroids must be dissociated into single cells for flow cytometry analysis, the physiological relevance of the 3D grown cells enable more accurate data than data obtained from cells grown in 2D.
2. Safe, reliable & automation friendly solution for 3D culturing before analysis
Gain additional sales by expanding your consumable collection to include more than just fluorescent dyes. Bring more value to your customers by offering matrices well suitable for culturing before flow cytometry analysis and post cell sorting.
When harvesting cells from the cultures, our cellulase enzyme has shown to not affect cell structure, cell viability or cell function, thus making the enzymatic recovery of cells extremely efficient. The cellulase enzyme degrades only nanocellulose fibers. The nanocellulose fibers turn into soluble glucose and the semi-solid hydrogel breaks down into a liquid solution. The cultured cells will be free from the 3D cell culture environment for downstream processing.
Cell structure
In a study by Kanninen & Lou, the three-dimensional cell structure is retained after recovering them from our nanocellulose hydrogels. Scanning electron microscopy (SEM) images of HepG2 spheroids revealing microvilli structure typical for hepatocytes.
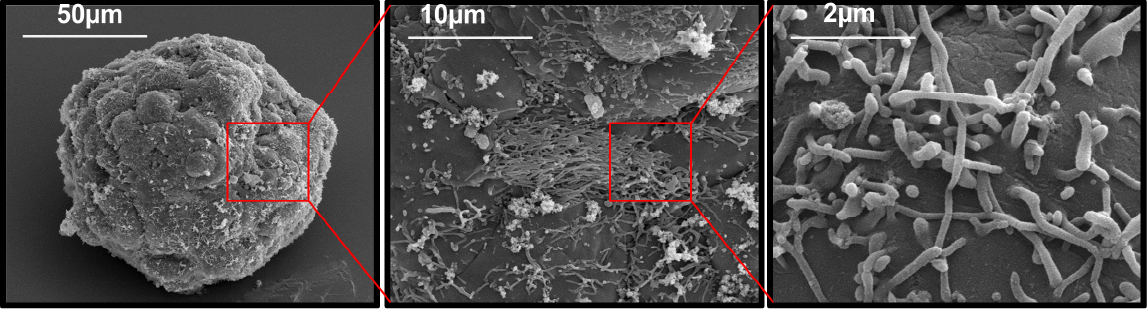
Images from Liisa Kanninen and Yan-Ru Lou, University of Helsinki.
Cell function
Even the most sensitive cell types, like induced pluripotent stem cells and embryonic stem cells are not harmed by our enzymatic recovery. In a study by Lou, both of the cells exhibited typical morphology and expressed the pluripotency markers OCT4 and SSEA-4.
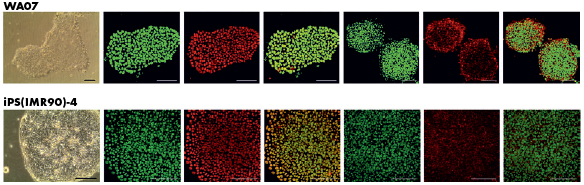
Image adapted from Lou, Y.-R. et al., (2014).
Cell viability
In a study, fibroblasts were cultured in 0.4% nanofibrillated cellulose (NFC) at 1x10^6cells/ml for 24hrs then cellulase enzyme degradation was performed. Cellulase enzyme was used at 300, 600 and 900 μg/mg to degrade the NFC. The RealTime-Glo™ viability assay was used to determinate the cell viability over 20 hours of culture.
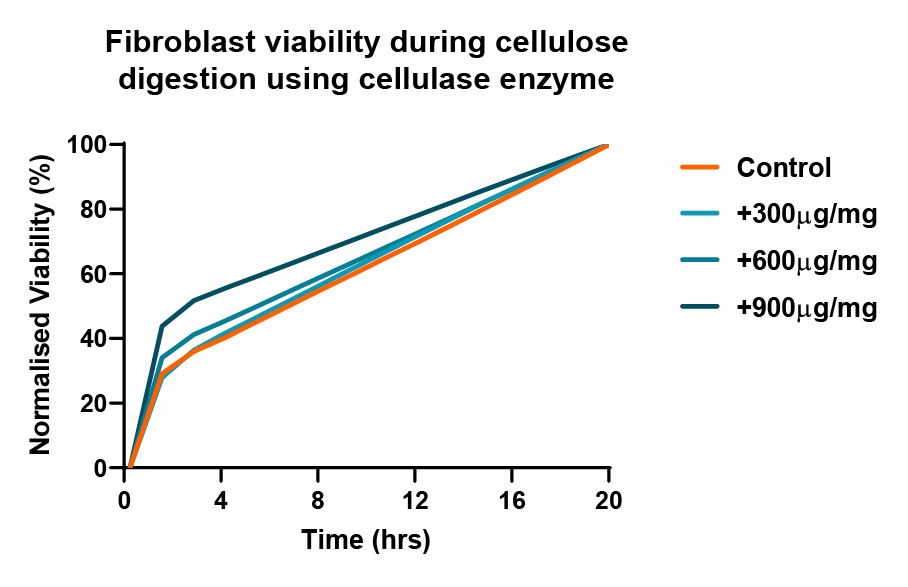
Unlike animal-based matrices on the markets, our hydrogels do not contain any unknown components that may leave residual on the sample. Such residual may interfere and even falsify the results, as there is no certainty of whether the animal-based matrix influenced where the fluorescent markers bind to.
Our hydrogels contain only wood nanofibrillar cellulose fibers and ultra-pure water. In addition to the two main components of our hydrogels, the user can mix their components of choice (proteins, growth factors etc.). This way the customer stays in full control of the cell culture environment and its components.
See video, how our hydrogels can be used for setting up 3D cell culture assays.
Nanofibrillar cellulose works well in high throughput assays. NFC can be dispensed in room temperature, which reduces the need for complex temperature control steps, such as cooling down the instruments and reagents. Furthermore, with NFC there is no need for mechanical steps to break-down the hydrogels to recover your cells. Rather, simply use liquid handling to dispense our propriety cellulase enzyme solution and recover your cells.
3. 3D culturing after FACS (Fluorescence-activated Cell Sorting)
Provide an easy solution for your customers: offer same cell culture matrix throughout the culture process, both before analysis and after cell sorting.
Once cells have been sorted with a flow cytometer, nanofibrillar cellulose can be used for culturing the homogenous cell populations for future characterization and research. Nanofibrillar cellulose acts as a scaffold for cells, increasing the structural similarity of cultured cells compared to cells growing in human body.
Would you like to learn more about nanofibrillar cellulose?
We are soon launching new information about NFC. Fill in the form so you can be among the first ones to be notified!